A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC
- PMID: 29874546
- PMCID: PMC6063769
- DOI: 10.1016/j.jtho.2018.03.035
A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC
Abstract
Background: Despite the significant antitumor activity of pembrolizumab in NSCLC, clinical benefit has been less frequently observed in patients whose tumors harbor EGFR mutations compared to EGFR wild-type patients. Our single-center experience on the KEYNOTE-001 trial suggested that pembrolizumab-treated EGFR-mutant patients, who were tyrosine kinase inhibitor (TKI) naïve, had superior clinical outcomes to those previously treated with a TKI. As TKI naïve EGFR-mutants have generally been excluded from pembrolizumab studies, data to guide treatment decisions in this patient population is lacking, particularly in patients with programmed death ligand 1 (PD-L1) expression ≥50%.
Methods: We conducted a phase II trial (NCT02879994) of pembrolizumab in TKI naive patients with EGFR mutation-positive, advanced NSCLC and PD-L1-positive (≥1%, 22C3 antibody) tumors. Pembrolizumab was administered 200 mg every 3 weeks. The primary endpoint was objective response rate. Secondary endpoints included safety of pembrolizumab, additional pembrolizumab efficacy endpoints, and efficacy and safety of an EGFR TKI after pembrolizumab.
Results: Enrollment was ceased due to lack of efficacy after 11 of 25 planned patients were treated. Eighty-two percent of trial patients were treatment naïve, 64% had sensitizing EGFR mutations, and 73% had PD-L1 expression ≥50%. Only 1 patient had an objective response (9%), but repeat analysis of this patient's tumor definitively showed the original report of an EGFR mutation to be erroneous. Observed treatment-related adverse events were similar to prior experience with pembrolizumab, but two deaths within 6 months of enrollment, including one attributed to pneumonitis, were of concern.
Conclusions: Pembrolizumab's lack of efficacy in TKI naïve, PD-L1+, EGFR-mutant patients with advanced NSCLC, including those with PD-L1 expression ≥50%, suggests that it is not an appropriate therapeutic choice in this setting.
Keywords: EGFR; NSCLC; pembrolizumab; programmed death 1 (PD-1); programmed death ligand 1; tumor immunology.
Copyright © 2018 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Lisberg reports compensated AstraZeneca advisory board attendance.
Dr. Garon reports funds to his institution from AstraZenca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Genentech, Mirati, Merck, and Novartis.
Dr. Goldman reports grants from BMS and Medimmune/AstraZeneca during the conduct of the study.
Dr. Mendenhall reports compensation for Merck speaker’s bureau.
The remaining authors have no conflicts of interest to report.
Figures
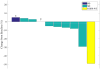

Comment in
-
Impact of PD-L1 Expression in EGFR-Positive NSCLC? The Answer Remains the Same….J Thorac Oncol. 2018 Aug;13(8):1060-1061. doi: 10.1016/j.jtho.2018.06.001. Epub 2018 Jul 2. J Thorac Oncol. 2018. PMID: 30056859 No abstract available.
-
Immunotherapies in the management of epidermal growth factor receptor mutated non-small cell lung cancer: a role will be found?Transl Lung Cancer Res. 2018 Dec;7(Suppl 4):S370-S372. doi: 10.21037/tlcr.2018.09.18. Transl Lung Cancer Res. 2018. PMID: 30705859 Free PMC article. No abstract available.
-
Immunotherapy in tyrosine kinase inhibitor-naïve advanced epidermal growth factor receptor-mutant non-small cell lung cancer-driving down a precarious road in driver-mutated lung cancer.Transl Lung Cancer Res. 2018 Dec;7(Suppl 4):S377-S380. doi: 10.21037/tlcr.2018.09.16. Transl Lung Cancer Res. 2018. PMID: 30705861 Free PMC article. No abstract available.
-
Role of immune checkpoint blockers in patients with EGFR mutation.Transl Lung Cancer Res. 2018 Dec;7(Suppl 4):S385-S387. doi: 10.21037/tlcr.2018.09.13. Transl Lung Cancer Res. 2018. PMID: 30705863 Free PMC article. No abstract available.
-
Is an immune checkpoint inhibitor really a hopeless therapeutic choice for EGFR-mutant non-small cell lung cancer (NSCLC) patients?Ann Transl Med. 2019 Mar;7(Suppl 1):S32. doi: 10.21037/atm.2019.02.18. Ann Transl Med. 2019. PMID: 31032311 Free PMC article. No abstract available.
-
Is the game over for PD-1 inhibitors in EGFR mutant non-small cell lung cancer?Transl Lung Cancer Res. 2019 Dec;8(Suppl 4):S339-S342. doi: 10.21037/tlcr.2019.04.09. Transl Lung Cancer Res. 2019. PMID: 32038910 Free PMC article. No abstract available.
References
-
- Garon EB, et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;372(21):2018–2028. - PubMed
-
- Hellmann MD, et al. MINI03.05 Efficacy of Pembrolizumab in Key Subgroups of Patients with Advanced NSCLC. Journal of Thoracic Oncology. 2015;10(9):S261–S406.
-
- Sharma SV, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

