Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States
- PMID: 29874566
- PMCID: PMC6039826
- DOI: 10.1016/j.cmet.2018.05.011
Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States
Abstract
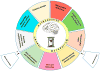
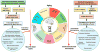
During aging, the cellular milieu of the brain exhibits tell-tale signs of compromised bioenergetics, impaired adaptive neuroplasticity and resilience, aberrant neuronal network activity, dysregulation of neuronal Ca2+ homeostasis, the accrual of oxidatively modified molecules and organelles, and inflammation. These alterations render the aging brain vulnerable to Alzheimer's and Parkinson's diseases and stroke. Emerging findings are revealing mechanisms by which sedentary overindulgent lifestyles accelerate brain aging, whereas lifestyles that include intermittent bioenergetic challenges (exercise, fasting, and intellectual challenges) foster healthy brain aging. Here we provide an overview of the cellular and molecular biology of brain aging, how those processes interface with disease-specific neurodegenerative pathways, and how metabolic states influence brain health.
Keywords: Alzheimer’s disease; Parkinson’s disease; aging; amyloid; autophagic; hippocampus; ketones; mitochondrial dysfunction; synaptic dysfunction; synuclein.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

