Pharmacodynamic Effects of a 6-Hour Regimen of Enoxaparin in Patients Undergoing Primary Percutaneous Coronary Intervention (PENNY PCI Study)
- PMID: 29874689
- PMCID: PMC6202933
- DOI: 10.1055/s-0038-1657768
Pharmacodynamic Effects of a 6-Hour Regimen of Enoxaparin in Patients Undergoing Primary Percutaneous Coronary Intervention (PENNY PCI Study)
Abstract
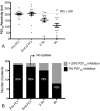
Delayed onset of action of oral P2Y12 inhibitors in ST-elevation myocardial infarction (STEMI) patients may increase the risk of acute stent thrombosis. Available parenteral anti-thrombotic strategies, to deal with this issue, are limited by added cost and increased risk of bleeding. We investigated the pharmacodynamic effects of a novel regimen of enoxaparin in STEMI patients undergoing primary percutaneous coronary intervention (PPCI). Twenty patients were recruited to receive 0.75 mg/kg bolus of enoxaparin (pre-PPCI) followed by infusion of enoxaparin 0.75 mg/kg/6 h. At four time points (pre-anti-coagulation, end of PPCI, 2-3 hours into infusion and at the end of infusion), anti-Xa levels were determined using chromogenic assays, fibrin clots were assessed by turbidimetric analysis and platelet P2Y12 inhibition was determined by VerifyNow P2Y12 assay. Clinical outcomes were determined 14 hours after enoxaparin initiation. Nineteen of 20 patients completed the enoxaparin regimen; one patient, who developed no-reflow phenomenon, was switched to tirofiban after the enoxaparin bolus. All received ticagrelor 180 mg before angiography. Mean (± standard error of the mean) anti-Xa levels were sustained during enoxaparin infusion (1.17 ± 0.06 IU/mL at the end of PPCI and 1.003 ± 0.06 IU/mL at 6 hours), resulting in prolonged fibrin clot lag time and increased lysis potential. Onset of platelet P2Y12 inhibition was delayed in opiate-treated patients. No patients had thrombotic or bleeding complications. In conclusion, enoxaparin 0.75 mg/kg bolus followed by 0.75 mg/kg/6 h provides sustained anti-Xa levels in PPCI patients. This may protect from acute stent thrombosis in opiate-treated PPCI patients who frequently have delayed onset of oral P2Y12 inhibition.
Georg Thieme Verlag KG Stuttgart · New York.
Conflict of interest statement
R.F.S. reports research grants from AstraZeneca, PlaqueTec; Honoraria from AstraZeneca; Consultant/Advisory Board fees from AstraZeneca, Actelion, Avacta, Bayer, Bristol Myers Squibb/Pfizer, Idorsia, Novartis and The Medicines Company. Other authors have no disclosures.
Figures


References
-
- Ibanez B, James S, Agewall S et al.2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(02):119–177. - PubMed
-
- Wiviott S D, Braunwald E, McCabe C H et al.Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. - PubMed
-
- Wallentin L, Becker R C, Budaj A et al.Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. - PubMed
-
- Wiviott S D, Trenk D, Frelinger A L et al.Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116(25):2923–2932. - PubMed
-
- Gurbel P A, Bliden K P, Butler K et al.Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577–2585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

