Tubular and Spherical SiO₂ Obtained by Sol Gel Method for Lipase Immobilization and Enzymatic Activity
- PMID: 29874881
- PMCID: PMC6100421
- DOI: 10.3390/molecules23061362
Tubular and Spherical SiO₂ Obtained by Sol Gel Method for Lipase Immobilization and Enzymatic Activity
Abstract
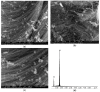
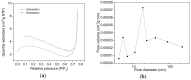
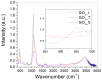
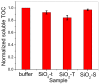
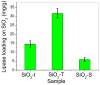
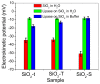
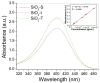
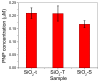
A wide range of hybrid biomaterials has been designed in order to sustain bioremediation processes by associating sol-gel SiO₂ matrices with various biologically active compounds (enzymes, antibodies). SiO₂ is a widespread, chemically stable and non-toxic material; thus, the immobilization of enzymes on silica may lead to improving the efficiency of biocatalysts in terms of endurance and economic costs. Our present work explores the potential of different hybrid morphologies, based on hollow tubes and solid spheres of amorphous SiO₂, for enzyme immobilization and the development of competitive biocatalysts. The synthesis protocol and structural characterization of spherical and tubular SiO₂ obtained by the sol gel method were fully investigated in connection with the subsequent immobilization of lipase from Rhizopus orizae. The immobilization is conducted at pH 6, lower than the isoelectric point of lipase and higher than the isoelectric point of silica, which is meant to sustain the physical interactions of the enzyme with the SiO₂ matrix. The morphological, textural and surface properties of spherical and tubular SiO₂ were investigated by SEM, nitrogen sorption, and electrokinetic potential measurements, while the formation and characterization of hybrid organic-inorganic complexes were studied by UV-VIS, FTIR-ATR and fluorescence spectroscopy. The highest degree of enzyme immobilization (as depicted from total organic carbon) was achieved for tubular morphology and the hydrolysis of p-nitrophenyl acetate was used as an enzymatic model reaction conducted in the presence of hybrid lipase⁻SiO₂ complex.
Keywords: SiO2; enzymatic catalysis; lipase immobilization; tubular and spherical morphology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Parvulescu V., Anastasescu C., Su B.L. Highly selective oxidation of aromatic hydrocarbons (Styrene, Benzene and Toluene) with H2O2 over Ni, Ni-Cr and Ni-Ru modified MCM-41 catalysts. Stud. Surf. Sci. Catal. 2002;142:1213–1220.
-
- Sanchez C., Julián B., Belleville P., Popall M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005;15:3559–3592. doi: 10.1039/b509097k. - DOI
-
- Yu W.H., Fang M., Tong D.S., Shao P., Xu T.N., Zhou C.H. Immobilization of Candida rugosa lipase on hexagonal mesoporous silca and selective estrification in nonaqueous medium. Biochem. Eng. J. 2013;70:97–105. doi: 10.1016/j.bej.2012.10.005. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

