Translational Model of Zika Virus Disease in Baboons
- PMID: 29875247
- PMCID: PMC6069201
- DOI: 10.1128/JVI.00186-18
Translational Model of Zika Virus Disease in Baboons
Abstract
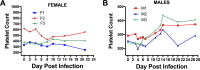
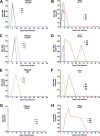
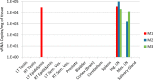
Zika virus (ZIKV) is an emerging mosquito-borne flavivirus with devastating outcomes seen recently in the Americas due to the association of maternal ZIKV infection with fetal microcephaly and other fetal malformations not previously associated with flavivirus infections. Here, we have developed the olive baboon (Papio anubis) as a nonhuman primate (NHP) translational model for the study of ZIKV pathogenesis and associated disease outcomes to contrast and compare with humans and other major NHPs, such as macaques. Following subcutaneous inoculation of adult male and nonpregnant female baboons, viremia was detected at 3 and 4 days postinfection (dpi) with the concordant presentation of a visible rash and conjunctivitis, similar to human ZIKV infection. Furthermore, virus was detected in the mucosa and cerebrospinal fluid. A robust ZIKV-specific IgM and IgG antibody response was also observed in all the animals. These data show striking similarity between humans and the olive baboon following infection with ZIKV, suggesting our model is a suitable translational NHP model to study ZIKV pathogenesis and potential therapeutics.IMPORTANCE ZIKV was first identified in 1947 in a sentinel rhesus monkey in Uganda and subsequently spread to Southeast Asia. Until 2007, only a small number of cases were reported, and ZIKV infection was relatively minor until the South Pacific and Brazilian outbreaks, where more severe outcomes were reported. Here, we present the baboon as a nonhuman primate model for contrast and comparison with other published animal models of ZIKV, such as the mouse and macaque species. Baboons breed year round and are not currently a primary nonhuman primate species used in biomedical research, making them more readily available for studies other than human immunodeficiency virus studies, which many macaque species are designated for. This, taken together with the similarities baboons have with humans, such as immunology, reproduction, genetics, and size, makes the baboon an attractive NHP model for ZIKV studies in comparison to other nonhuman primates.
Keywords: West Nile virus; ZIKV; Zika virus; baboon; flavivirus; nonhuman primate.
Copyright © 2018 American Society for Microbiology.
Figures




Similar articles
-
Maternal Zika Virus (ZIKV) Infection following Vaginal Inoculation with ZIKV-Infected Semen in Timed-Pregnant Olive Baboons.J Virol. 2020 May 18;94(11):e00058-20. doi: 10.1128/JVI.00058-20. Print 2020 May 18. J Virol. 2020. PMID: 32188737 Free PMC article.
-
Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon.PLoS Pathog. 2019 Jan 18;15(1):e1007507. doi: 10.1371/journal.ppat.1007507. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30657788 Free PMC article.
-
Zika Virus Infection, Reproductive Organ Targeting, and Semen Transmission in the Male Olive Baboon.J Virol. 2019 Dec 12;94(1):e01434-19. doi: 10.1128/JVI.01434-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597777 Free PMC article.
-
Zika virus: A new threat to human reproduction.Am J Reprod Immunol. 2017 Feb;77(2). doi: 10.1111/aji.12614. Epub 2016 Dec 14. Am J Reprod Immunol. 2017. PMID: 27966802 Review.
-
Using Macaques to Address Critical Questions in Zika Virus Research.Annu Rev Virol. 2019 Sep 29;6(1):481-500. doi: 10.1146/annurev-virology-092818-015732. Epub 2019 Jun 10. Annu Rev Virol. 2019. PMID: 31180813 Free PMC article. Review.
Cited by
-
Molecular Approaches for the Validation of the Baboon as a Nonhuman Primate Model for the Study of Zika Virus Infection.Front Cell Infect Microbiol. 2022 Apr 14;12:880860. doi: 10.3389/fcimb.2022.880860. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35493734 Free PMC article.
-
Maternal Zika Virus (ZIKV) Infection following Vaginal Inoculation with ZIKV-Infected Semen in Timed-Pregnant Olive Baboons.J Virol. 2020 May 18;94(11):e00058-20. doi: 10.1128/JVI.00058-20. Print 2020 May 18. J Virol. 2020. PMID: 32188737 Free PMC article.
-
Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon.PLoS Pathog. 2019 Jan 18;15(1):e1007507. doi: 10.1371/journal.ppat.1007507. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30657788 Free PMC article.
-
Pathophysiology and Mechanisms of Zika Virus Infection in the Nervous System.Annu Rev Neurosci. 2019 Jul 8;42:249-269. doi: 10.1146/annurev-neuro-080317-062231. Annu Rev Neurosci. 2019. PMID: 31283901 Free PMC article. Review.
-
Experimental Infection of Pregnant Female Sheep with Zika Virus During Early Gestation.Viruses. 2019 Aug 29;11(9):795. doi: 10.3390/v11090795. Viruses. 2019. PMID: 31470560 Free PMC article.
References
-
- Brasil P, Pereira JP Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baiao AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. 2016. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 375:2321–2334. doi:10.1056/NEJMoa1602412. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

