A model for postzygotic mosaicisms quantifies the allele fraction drift, mutation rate, and contribution to de novo mutations
- PMID: 29875290
- PMCID: PMC6028137
- DOI: 10.1101/gr.230003.117
A model for postzygotic mosaicisms quantifies the allele fraction drift, mutation rate, and contribution to de novo mutations
Abstract
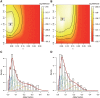
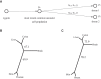
The allele fraction (AF) distribution, occurrence rate, and evolutionary contribution of postzygotic single-nucleotide mosaicisms (pSNMs) remain largely unknown. In this study, we developed a mathematical model to describe the accumulation and AF drift of pSNMs during the development of multicellular organisms. By applying the model, we quantitatively analyzed two large-scale data sets of pSNMs identified from human genomes. We found that the postzygotic mutation rate per cell division during early embryogenesis, especially during the first cell division, was higher than the average mutation rate in either male or female gametes. We estimated that the stochastic cell death rate per cell cleavage during human embryogenesis was ∼5%, and parental pSNMs occurring during the first three cell divisions contributed to ∼10% of the de novo mutations observed in children. We further demonstrated that the genomic profiles of pSNMs could be used to measure the divergence distance between tissues. Our results highlight the importance of pSNMs in estimating recurrence risk and clarified the quantitative relationship between postzygotic and de novo mutations.
© 2018 Ye et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

References
-
- Abyzov A, Tomasini L, Zhou B, Vasmatzis N, Coppola G, Amenduni M, Pattni R, Wilson M, Gerstein M, Weissman S, et al. 2017. One thousand somatic SNVs per skin fibroblast cell set baseline of mosaic mutational load with patterns that suggest proliferative origin. Genome Res 27: 512–523. - PMC - PubMed
-
- Argani P, Walsh PC, Epstein JI. 1998. Analysis of the prostatic central zone in patients with unilateral absence of wolffian duct structures: further evidence of the mesodermal origin of the prostatic central zone. J Urol 160: 2126–2129. - PubMed
-
- Bernkopf M, Hunt D, Koelling N, Morgan T, Collins AL, Fairhurst J, Robertson SP, Douglas AGL, Goriely A. 2017. Quantification of transmission risk in a male patient with a FLNB mosaic mutation causing Larsen syndrome: implications for genetic counseling in postzygotic mosaicism cases. Hum Mutat 38: 1360–1364. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
