Intracapillary immune complexes recruit and activate slan-expressing CD16+ monocytes in human lupus nephritis
- PMID: 29875315
- PMCID: PMC6124405
- DOI: 10.1172/jci.insight.96492
Intracapillary immune complexes recruit and activate slan-expressing CD16+ monocytes in human lupus nephritis
Abstract
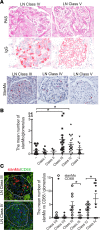
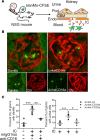
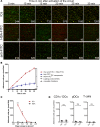
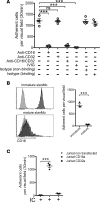
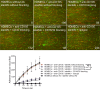
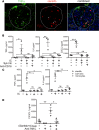
Lupus nephritis is a major cause of morbidity in patients with systemic lupus erythematosus. Among the different types of lupus nephritis, intracapillary immune complex (IC) deposition and accumulation of monocytes are hallmarks of lupus nephritis class III and IV. The relevance of intracapillary ICs in terms of monocyte recruitment and activation, as well as the nature and function of these monocytes are not well understood. For the early focal form of lupus nephritis (class III) we demonstrate a selective accumulation of the proinflammatory population of 6-sulfo LacNAc+ (slan) monocytes (slanMo), which locally expressed TNF-α. Immobilized ICs induced a direct recruitment of slanMo from the microcirculation via interaction with Fc γ receptor IIIA (CD16). Interestingly, intravenous immunoglobulins blocked CD16 and prevented cell recruitment. Engagement of immobilized ICs by slanMo induced the production of neutrophil-attracting chemokine CXCL2 as well as TNF-α, which in a forward feedback loop stimulated endothelial cells to produce the slanMo-recruiting chemokine CX3CL1 (fractalkine). In conclusion, we observed that expression of CD16 equips slanMo with a unique capacity to orchestrate early IC-induced inflammatory responses in glomeruli and identified slanMo as a pathogenic proinflammatory cell type in lupus nephritis.
Keywords: Autoimmunity; Immunology; Monocytes.
Conflict of interest statement
Figures
Similar articles
-
Fractalkine expression and CD16+ monocyte accumulation in glomerular lesions: association with their severity and diversity in lupus models.Am J Physiol Renal Physiol. 2010 Jul;299(1):F207-16. doi: 10.1152/ajprenal.00482.2009. Epub 2010 Apr 21. Am J Physiol Renal Physiol. 2010. PMID: 20410215
-
Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis.Am J Kidney Dis. 2007 Jul;50(1):47-58. doi: 10.1053/j.ajkd.2007.04.012. Am J Kidney Dis. 2007. PMID: 17591524
-
Current Concepts on 6-sulfo LacNAc Expressing Monocytes (slanMo).Front Immunol. 2019 May 22;10:948. doi: 10.3389/fimmu.2019.00948. eCollection 2019. Front Immunol. 2019. PMID: 31191513 Free PMC article. Review.
-
Increased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus.Arthritis Res Ther. 2009;11(1):R6. doi: 10.1186/ar2590. Epub 2009 Jan 14. Arthritis Res Ther. 2009. PMID: 19144150 Free PMC article.
-
Histopathology of lupus nephritis.Clin Rev Allergy Immunol. 2011 Jun;40(3):170-80. doi: 10.1007/s12016-010-8207-1. Clin Rev Allergy Immunol. 2011. PMID: 20514528 Review.
Cited by
-
The role of lung-restricted autoantibodies in the development of primary and chronic graft dysfunction.Front Transplant. 2023 Nov 9;2:1237671. doi: 10.3389/frtra.2023.1237671. eCollection 2023. Front Transplant. 2023. PMID: 38993924 Free PMC article. Review.
-
Immune complex-induced haptokinesis in human non-classical monocytes.Front Immunol. 2023 Mar 1;14:1078241. doi: 10.3389/fimmu.2023.1078241. eCollection 2023. Front Immunol. 2023. PMID: 36936904 Free PMC article.
-
From therapeutic antibodies to immune complex vaccines.NPJ Vaccines. 2019 Jan 17;4:2. doi: 10.1038/s41541-018-0095-z. eCollection 2019. NPJ Vaccines. 2019. PMID: 30675393 Free PMC article. Review.
-
Induction of NF-κB inflammatory pathway in monocytes by microparticles from patients with systemic lupus erythematosus.Heliyon. 2020 Dec 23;6(12):e05815. doi: 10.1016/j.heliyon.2020.e05815. eCollection 2020 Dec. Heliyon. 2020. PMID: 33409392 Free PMC article.
-
Neoadjuvant Radiochemotherapy Significantly Alters the Phenotype of Plasmacytoid Dendritic Cells and 6-Sulfo LacNAc+ Monocytes in Rectal Cancer.Front Immunol. 2019 Mar 29;10:602. doi: 10.3389/fimmu.2019.00602. eCollection 2019. Front Immunol. 2019. PMID: 30984181 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

