Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury
- PMID: 29875326
- PMCID: PMC6124442
- DOI: 10.1172/jci.insight.120137
Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury
Abstract
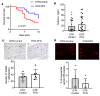
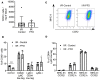
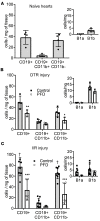
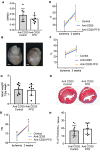
Despite the long-standing recognition that the immune response to acute myocardial injury contributes to adverse left ventricular (LV) remodeling, it has not been possible to effectively target this clinically. Using 2 different in vivo models of acute myocardial injury, we show that pirfenidone confers beneficial effects in the murine heart through an unexpected mechanism that depends on cardiac B lymphocytes. Naive hearts contained a large population of CD19+CD11b-CD23-CD21-IgD+IgMlo lymphocytes, and 2 smaller populations of CD19+CD11b+ B1a and B1b cells. In response to tissue injury, there was an increase in neutrophils, monocytes, macrophages, as well as an increase in CD19+ CD11b- B lymphocytes. Treatment with pirfenidone had no effect on the number of neutrophils, monocytes, or macrophages, but decreased CD19+CD11b- lymphocytes. B cell depletion abrogated the beneficial effects of pirfenidone. In vitro studies demonstrated that stimulation with lipopolysaccharide and extracts from necrotic cells activated CD19+ lymphocytes through a TIRAP-dependent pathway. Treatment with pirfenidone attenuated this activation of B cells. These findings reveal a previously unappreciated complexity of myocardial B lymphocytes within the inflammatory infiltrate triggered by cardiac injury and suggest that pirfenidone exerts beneficial effects in the heart through a unique mechanism that involves modulation of cardiac B lymphocytes.
Keywords: B cells; Cardiology; Heart failure.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

