A Novel Porcine Model for Future Studies of Cell-enriched Fat Grafting
- PMID: 29876178
- PMCID: PMC5977937
- DOI: 10.1097/GOX.0000000000001735
A Novel Porcine Model for Future Studies of Cell-enriched Fat Grafting
Abstract
Background: Cell-enriched fat grafting has shown promising results for improving graft survival, although many questions remain unanswered. A large animal model is crucial for bridging the gap between rodent studies and human trials. We present a step-by-step approach in using the Göttingen minipig as a model for future studies of cell-enriched large volume fat grafting.
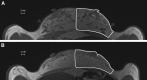
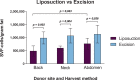
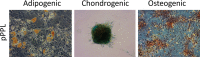
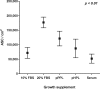
Methods: Fat grafting was performed as bolus injections and structural fat grafting. Graft retention was assessed by magnetic resonance imaging after 120 days. The stromal vascular fraction (SVF) was isolated from excised fat and liposuctioned fat from different anatomical sites and analyzed. Porcine adipose-derived stem/stromal cells (ASCs) were cultured in different growth supplements, and population doubling time, maximum cell yield, expression of surface markers, and differentiation potential were investigated.
Results: Structural fat grafting in the breast and subcutaneous bolus grafting in the abdomen revealed average graft retention of 53.55% and 15.28%, respectively, which are similar to human reports. Liposuction yielded fewer SVF cells than fat excision, and abdominal fat had the most SVF cells/g fat with SVF yields similar to humans. Additionally, we demonstrated that porcine ASCs can be readily isolated and expanded in culture in allogeneic porcine platelet lysate and fetal bovine serum and that the use of 10% porcine platelet lysate or 20% fetal bovine serum resulted in population doubling time, maximum cell yield, surface marker profile, and trilineage differentiation that were comparable with humans.
Conclusions: The Göttingen minipig is a feasible and cost-effective, large animal model for future translational studies of cell-enriched fat grafting.
Figures



Similar articles
-
Cell-Enriched Fat Grafting Improves Graft Retention in a Porcine Model: A Dose-Response Study of Adipose-Derived Stem Cells versus Stromal Vascular Fraction.Plast Reconstr Surg. 2019 Sep;144(3):397e-408e. doi: 10.1097/PRS.0000000000005920. Plast Reconstr Surg. 2019. PMID: 31461016
-
Harvesting technique affects adipose-derived stem cell yield.Aesthet Surg J. 2015 May;35(4):467-76. doi: 10.1093/asj/sju055. Epub 2015 Mar 18. Aesthet Surg J. 2015. PMID: 25791999 Free PMC article.
-
Stem Cell Therapy Enriched Fat Grafting for the Reconstruction of Craniofacial Deficits.Plast Reconstr Surg Glob Open. 2023 Jun 19;11(6):e5056. doi: 10.1097/GOX.0000000000005056. eCollection 2023 Jun. Plast Reconstr Surg Glob Open. 2023. PMID: 37342306 Free PMC article.
-
The Safety and Efficacy of Cell-Assisted Fat Grafting to Traditional Fat Grafting in the Anterior Mid-Face: An Indirect Assessment by 3D Imaging.Aesthetic Plast Surg. 2015 Dec;39(6):833-46. doi: 10.1007/s00266-015-0533-5. Epub 2015 Sep 3. Aesthetic Plast Surg. 2015. PMID: 26335660
-
Adult adipose-derived stem cells and breast cancer: a controversial relationship.Springerplus. 2014 Jul 8;3:345. doi: 10.1186/2193-1801-3-345. eCollection 2014. Springerplus. 2014. PMID: 25089245 Free PMC article. Review.
Cited by
-
Adipose stromal vascular fraction: a promising treatment for severe burn injury.Hum Cell. 2022 Sep;35(5):1323-1337. doi: 10.1007/s13577-022-00743-z. Epub 2022 Jul 30. Hum Cell. 2022. PMID: 35906507 Review.
-
Fat extract improves fat graft survival via proangiogenic, anti-apoptotic and pro-proliferative activities.Stem Cell Res Ther. 2019 Jun 13;10(1):174. doi: 10.1186/s13287-019-1290-1. Stem Cell Res Ther. 2019. PMID: 31196213 Free PMC article.
References
-
- Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28:111–119.. - PubMed
-
- Kanchwala SK, Holloway L, Bucky LP. Reliable soft tissue augmentation: a clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Ann Plast Surg. 2005;55:30–35.; discussion 35. - PubMed
-
- Khouri R, Del Vecchio D. Breast reconstruction and augmentation using pre-expansion and autologous fat transplantation. Clin Plast Surg. 2009;36:269–80, viii.. - PubMed
-
- Khouri RK, Smit JM, Cardoso E, et al. Percutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction? Plast Reconstr Surg. 2013;132:1280–1290.. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
