Detection of Central Nervous System Infiltration by Myeloid and Lymphoid Hematologic Neoplasms Using Flow Cytometry Analysis: Diagnostic Accuracy Study
- PMID: 29876349
- PMCID: PMC5974033
- DOI: 10.3389/fmed.2018.00070
Detection of Central Nervous System Infiltration by Myeloid and Lymphoid Hematologic Neoplasms Using Flow Cytometry Analysis: Diagnostic Accuracy Study
Abstract
Introduction: Infiltration of the central nervous system (CNS) by hematologic or lymphoid malignant cells can cause extensive neurological damage, be progressive and fatal. However, usually, the cerebrospinal fluid (CSF) has low cellularity and rapid cell degeneration, which can impair cytometry analysis. Storage and transport measures, sample preparation, and staining protocols can interfere with diagnostic accuracy.
Objective: To calculate the diagnostic performance of flow cytometry (FC) using a cell stabilizer for sample preservation compared to cytomorphology in the detection of CNS infiltration by lymphoid and hematologic neoplasms.
Methods: Cell samples from all consecutive patients with suspected infiltration by hematological malignancies evaluated between January 2014 and December 2016 were included. Cases were analyzed by FC using a cell preservation medium and cytomorphology. Sensitivity and specificity were calculated.
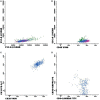
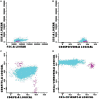
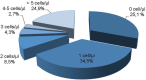
Results: From 414 CSF samples, 72 had a phenotype compatible with characteristics of infiltration by hematological disease, whereas cytology was positive for 35 cases. FC showed higher sensitivity and specificity when compared to cytomorphology, particularly in cases with cellularity under 5 leukocytes/mm3.
Conclusion: We demonstrated that collecting CSF in a medium that preserves the stability of the sample improves accuracy when compared to cytomorphology, particularly in low-volume and low-cellularity samples.
Keywords: central nervous system; cerebrospinal fluid; cytology; flow cytometry; neoplasms.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

