Industrializing Autologous Adoptive Immunotherapies: Manufacturing Advances and Challenges
- PMID: 29876351
- PMCID: PMC5974219
- DOI: 10.3389/fmed.2018.00150
Industrializing Autologous Adoptive Immunotherapies: Manufacturing Advances and Challenges
Abstract
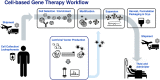
Cell therapy has proven to be a burgeoning field of investigation, evidenced by hundreds of clinical trials being conducted worldwide across a variety of cell types and indications. Many cell therapies have been shown to be efficacious in humans, such as modified T-cells and natural killer (NK) cells. Adoptive immunotherapy has shown the most promise in recent years, with particular emphasis on autologous cell sources. Chimeric Antigen Receptor (CAR)-based T-cell therapy targeting CD19-expressing B-cell leukemias has shown remarkable efficacy and reproducibility in numerous clinical trials. Recent marketing approval of Novartis' Kymriah™ (tisagenlecleucel) and Gilead/Kite's Yescarta™ (axicabtagene ciloleucel) by the FDA further underscores both the promise and legwork to be done if manufacturing processes are to become widely accessible. Further work is needed to standardize, automate, close, and scale production to bring down costs and democratize these and other cell therapies. Given the multiple processing steps involved, commercial-scale manufacturing of these therapies necessitates tighter control over process parameters. This focused review highlights some of the most recent advances used in the manufacturing of therapeutic immune cells, with a focus on T-cells. We summarize key unit operations and pain points around current manufacturing solutions. We also review emerging technologies, approaches and reagents used in cell isolation, activation, transduction, expansion, in-process analytics, harvest, cryopreservation and thaw, and conclude with a forward-look at future directions in the manufacture of adoptive immunotherapies.
Keywords: CAR T-cells; NK cell; autologous; bioreactor; cellular therapy; chimeric antigen receptor; immunotherapy manufacturing; scale-out.
Figures
References
-
- Automation of CAR-T cell adoptive immunotherapy bioprocessing: technology opportunities to debottleneck manufacturing (2016).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

