Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper [corrected]
- PMID: 29876802
- PMCID: PMC6132822
- DOI: 10.1007/s12311-018-0952-3
Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper [corrected]
Erratum in
-
Correction to: Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper.Cerebellum. 2018 Oct;17(5):683-684. doi: 10.1007/s12311-018-0959-9. Cerebellum. 2018. PMID: 29931663 Free PMC article.
Abstract
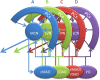
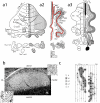
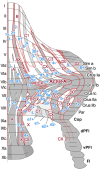
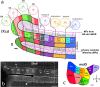
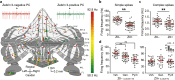
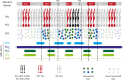
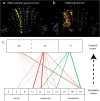
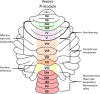
The compartmentalization of the cerebellum into modules is often used to discuss its function. What, exactly, can be considered a module, how do they operate, can they be subdivided and do they act individually or in concert are only some of the key questions discussed in this consensus paper. Experts studying cerebellar compartmentalization give their insights on the structure and function of cerebellar modules, with the aim of providing an up-to-date review of the extensive literature on this subject. Starting with an historical perspective indicating that the basis of the modular organization is formed by matching olivocorticonuclear connectivity, this is followed by consideration of anatomical and chemical modular boundaries, revealing a relation between anatomical, chemical, and physiological borders. In addition, the question is asked what the smallest operational unit of the cerebellum might be. Furthermore, it has become clear that chemical diversity of Purkinje cells also results in diversity of information processing between cerebellar modules. An additional important consideration is the relation between modular compartmentalization and the organization of the mossy fiber system, resulting in the concept of modular plasticity. Finally, examination of cerebellar output patterns suggesting cooperation between modules and recent work on modular aspects of emotional behavior are discussed. Despite the general consensus that the cerebellum has a modular organization, many questions remain. The authors hope that this joint review will inspire future cerebellar research so that we are better able to understand how this brain structure makes its vital contribution to behavior in its most general form.
Keywords: Aldolase C; Cerebellum; Climbing fibers; Compartments; Functional organization; Longitudinal stripes; Microzones; Mossy fibers; Purkinje cells; Zebrin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

