Toll-like receptor 4 signalling regulates antibody response to adenoviral vector-based vaccines by imprinting germinal centre quality
- PMID: 29876918
- PMCID: PMC6142292
- DOI: 10.1111/imm.12957
Toll-like receptor 4 signalling regulates antibody response to adenoviral vector-based vaccines by imprinting germinal centre quality
Abstract
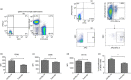
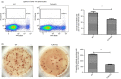
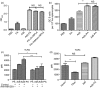
Adenoviral vectors (AdV) are considered promising candidates for vaccine applications. A prominent group of Toll-like receptors (TLRs) participate in the adenovirus-induced adaptive immune response, yet there is little information regarding the role of TLR4 in AdV-induced immune responses in recent literature. We investigated the function of TLR4 in both adaptive and innate immune responses to an AdV-based anthrax vaccine. By immunizing wild-type and TLR4 knockout (TLR4-KO) mice, we revealed the requirement of TLR4 in AdV-induced innate responses. We also showed that TLR4 functions are required for germinal centre responses in immunized mice, as expression of the apoptosis-related marker Fas was down-regulated on germinal centre B cells from TLR4-KO mice. Likewise, decreased expression of inducible costimulator on follicular T helper cells was observed in immunized TLR4-KO mice. Moreover, a potent protective antigen-specific humoral immune response was mimicked using an adjuvant system containing the TLR4 agonist monophosphoryl lipid A. Overall, our findings showed that very rapid antigen-specific antibody production is correlated with the TLR4-imprinted germinal centre response to AdV-based vaccine. These results provide additional evidence for the use of the AdV and a TLR agonist to induce humoral responses. Our findings offer new insights into rational vaccine design.
Keywords: Toll-like receptor 4 signalling; adenoviral vector-based vaccine; follicular T helper cell; germinal centre; humoral immunity.
© 2018 John Wiley & Sons Ltd.
Figures




Similar articles
-
Toll-Like Receptor 4 Regulates Rabies Virus-Induced Humoral Immunity through Recruitment of Conventional Type 2 Dendritic Cells to Lymph Organs.J Virol. 2021 Nov 23;95(24):e0082921. doi: 10.1128/JVI.00829-21. Epub 2021 Oct 6. J Virol. 2021. PMID: 34613801 Free PMC article.
-
Transient CD4+ T Cell Depletion Results in Delayed Development of Functional Vaccine-Elicited Antibody Responses.J Virol. 2016 Apr 14;90(9):4278-4288. doi: 10.1128/JVI.00039-16. Print 2016 May. J Virol. 2016. PMID: 26865713 Free PMC article.
-
B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers.J Immunol. 2014 Nov 1;193(9):4400-14. doi: 10.4049/jimmunol.1401720. Epub 2014 Sep 24. J Immunol. 2014. PMID: 25252960 Free PMC article.
-
Contribution of Toll-like receptor signaling to germinal center antibody responses.Immunol Rev. 2012 May;247(1):64-72. doi: 10.1111/j.1600-065X.2012.01115.x. Immunol Rev. 2012. PMID: 22500832 Free PMC article. Review.
-
The Role of TLR4 on B Cell Activation and Anti-β2GPI Antibody Production in the Antiphospholipid Syndrome.J Immunol Res. 2016;2016:1719720. doi: 10.1155/2016/1719720. Epub 2016 Oct 27. J Immunol Res. 2016. PMID: 27868072 Free PMC article. Review.
Cited by
-
Adenoviral Vector System: A Comprehensive Overview of Constructions, Therapeutic Applications and Host Responses.J Microbiol. 2024 Jul;62(7):491-509. doi: 10.1007/s12275-024-00159-4. Epub 2024 Jul 22. J Microbiol. 2024. PMID: 39037484 Review.
-
Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT).Semin Hematol. 2022 Apr;59(2):97-107. doi: 10.1053/j.seminhematol.2022.02.004. Epub 2022 Feb 23. Semin Hematol. 2022. PMID: 35512907 Free PMC article.
-
Toll-Like Receptor 4 Regulates Rabies Virus-Induced Humoral Immunity through Recruitment of Conventional Type 2 Dendritic Cells to Lymph Organs.J Virol. 2021 Nov 23;95(24):e0082921. doi: 10.1128/JVI.00829-21. Epub 2021 Oct 6. J Virol. 2021. PMID: 34613801 Free PMC article.
-
The use of adenoviral vectors in gene therapy and vaccine approaches.Genet Mol Biol. 2022 Oct 7;45(3 Suppl 1):e20220079. doi: 10.1590/1678-4685-GMB-2022-0079. eCollection 2022. Genet Mol Biol. 2022. PMID: 36206378 Free PMC article.
-
Intrinsic B cell TLR-BCR linked coengagement induces class-switched, hypermutated, neutralizing antibody responses in absence of T cells.Sci Adv. 2023 Apr 28;9(17):eade8928. doi: 10.1126/sciadv.ade8928. Epub 2023 Apr 28. Sci Adv. 2023. PMID: 37115935 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

