Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity
- PMID: 29877588
- PMCID: PMC6175443
- DOI: 10.1111/jnc.14482
Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity
Abstract
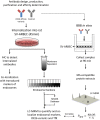
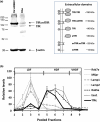
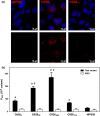
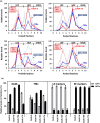
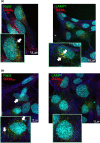
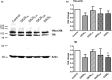
The blood-brain barrier (BBB) is a formidable obstacle to the delivery of therapeutics to the brain. Antibodies that bind transferrin receptor (TfR), which is enriched in brain endothelial cells, have been shown to cross the BBB and are being developed as fusion proteins to deliver therapeutic cargos to brain targets. Various antibodies have been developed for this purpose and their in vivo evaluation demonstrated that either low affinity or monovalent receptor binding re-directs their transcellular trafficking away from lysosomal degradation and toward improved exocytosis on the abluminal side of the BBB. However, these studies have been performed with antibodies that recognize different TfR epitopes and have different binding characteristics, preventing inter-study comparisons. In this study, the efficiency of transcytosis in vitro and intracellular trafficking in endosomal compartments were evaluated in an in vitro BBB model for affinity variants (Kd from 5 to174 nM) of the rat TfR-binding antibody, OX26. Distribution in subcellular fractions of the rat brain endothelial cells was determined using both targeted quantitative proteomics-selected reaction monitoring and fluorescent imaging with markers of early- and late endosomes. The OX26 variants with affinities of 76 and 108 nM showed improved trancytosis (Papp values) across the in vitro BBB model compared with a 5 nM OX26. Although ~40% of the 5 nM OX26 and ~35% of TfR co-localized with late-endosome/lysosome compartment, 76 and 108 nM affinity variants showed lower amounts in lysosomes and a predominant co-localization with early endosome markers. The study links bivalent TfR antibody affinity to mechanisms of sorting and trafficking away from late endosomes and lysosomes, resulting in improvement in their transcytosis efficiency.
Open practices: Open Science: This manuscript was awarded with the Open Materials Badge. For more information see: https://cos.io/our-services/open-science-badges/ Cover Image for this issue: doi: 10.1111/jnc.14193.
Keywords: affinity optimization; blood-brain barrier; intracellular trafficking; quantitative targeted proteomics; transferrin receptor antibody.
© 2018 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures

References
-
- Ahn J. and Johnstone R. M. (1993) Origin of a soluble truncated transferrin receptor. Blood 81, 2442–2451. - PubMed
-
- Artursson P. and Karlsson J. (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco‐2) cells. Biochem. Biophys. Res. Commun. 175, 880–885. - PubMed
-
- Cabezon I., Manich G., Martin‐Venegas R., Camins A., Pelegri C. and Vilaplana J. (2015) Trafficking of gold nanoparticles coated with the 8D3 anti‐transferrin receptor antibody at the mouse blood‐brain barrier. Mol. Pharm. 12, 4137–4145. - PubMed
-
- Chan L. N. and Gerhardt E. M. (1992) Transferrin receptor gene is hyperexpressed and transcriptionally regulated in differentiating erythroid cells. J. Biol. Chem. 267, 8254–8259. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

