Drug-Penetration Gradients Associated with Acquired Drug Resistance in Patients with Tuberculosis
- PMID: 29877726
- PMCID: PMC6221573
- DOI: 10.1164/rccm.201711-2333OC
Drug-Penetration Gradients Associated with Acquired Drug Resistance in Patients with Tuberculosis
Abstract
Rationale: Acquired resistance is an important driver of multidrug-resistant tuberculosis (TB), even with good treatment adherence. However, exactly what initiates the resistance and how it arises remain poorly understood.
Objectives: To identify the relationship between drug concentrations and drug susceptibility readouts (minimum inhibitory concentrations [MICs]) in the TB cavity.
Methods: We recruited patients with medically incurable TB who were undergoing therapeutic lung resection while on treatment with a cocktail of second-line anti-TB drugs. On the day of surgery, antibiotic concentrations were measured in the blood and at seven prespecified biopsy sites within each cavity. Mycobacterium tuberculosis was grown from each biopsy site, MICs of each drug identified, and whole-genome sequencing performed. Spearman correlation coefficients between drug concentration and MIC were calculated.
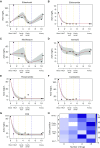
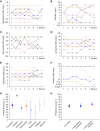
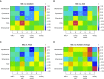
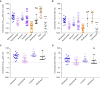
Measurements and main results: Fourteen patients treated for a median of 13 months (range, 5-31 mo) were recruited. MICs and drug resistance-associated single-nucleotide variants differed between the different geospatial locations within each cavity, and with pretreatment and serial sputum isolates, consistent with ongoing acquisition of resistance. However, pretreatment sputum MIC had an accuracy of only 49.48% in predicting cavitary MICs. There were large concentration-distance gradients for each antibiotic. The location-specific concentrations inversely correlated with MICs (P < 0.05) and therefore acquired resistance. Moreover, pharmacokinetic/pharmacodynamic exposures known to amplify drug-resistant subpopulations were encountered in all positions.
Conclusions: These data inform interventional strategies relevant to drug delivery, dosing, and diagnostics to prevent the development of acquired resistance. The role of high intracavitary penetration as a biomarker of antibiotic efficacy, when assessing new regimens, requires clarification.
Keywords: acquired drug resistance; drug gradient; lung cavity; sputum MIC; whole-genome sequencing.
Figures
References
-
- World Health Organization. United Nations high-level meeting on antimicrobial resistance. Geneva: World Health Organization, 2016 [accessed 2017 Nov 27]. Available from: http://www.un.org/pga/71/2016/09/21/press-release-hl-meeting-on-antimicr...
-
- Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. [online ahead of print] 15 Mar 2017; DOI: 10.1016/S2213-2600(17)30079-6. - PubMed
-
- Dheda K, Limberis JD, Pietersen E, Phelan J, Esmail A, Lesosky M, et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir Med. 2017;5:269–281. - PubMed
-
- Pietersen E, Ignatius E, Streicher EM, Mastrapa B, Padanilam X, Pooran A, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383:1230–1239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

