An expanded allosteric network in PTP1B by multitemperature crystallography, fragment screening, and covalent tethering
- PMID: 29877794
- PMCID: PMC6039181
- DOI: 10.7554/eLife.36307
An expanded allosteric network in PTP1B by multitemperature crystallography, fragment screening, and covalent tethering
Abstract
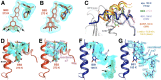
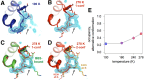
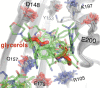
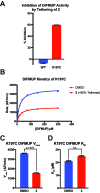
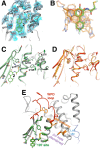
Allostery is an inherent feature of proteins, but it remains challenging to reveal the mechanisms by which allosteric signals propagate. A clearer understanding of this intrinsic circuitry would afford new opportunities to modulate protein function. Here, we have identified allosteric sites in protein tyrosine phosphatase 1B (PTP1B) by combining multiple-temperature X-ray crystallography experiments and structure determination from hundreds of individual small-molecule fragment soaks. New modeling approaches reveal 'hidden' low-occupancy conformational states for protein and ligands. Our results converge on allosteric sites that are conformationally coupled to the active-site WPD loop and are hotspots for fragment binding. Targeting one of these sites with covalently tethered molecules or mutations allosterically inhibits enzyme activity. Overall, this work demonstrates how the ensemble nature of macromolecular structure, revealed here by multitemperature crystallography, can elucidate allosteric mechanisms and open new doors for long-range control of protein function.
Keywords: E. coli; allostery; human; molecular biophysics; phosphatase; protein dynamics; structural biology.
© 2018, Keedy et al.
Conflict of interest statement
DK, ZH, JB, EK, TR, JB, NP, Fv, JW, JF No competing interests declared
Figures




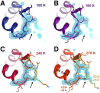

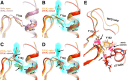
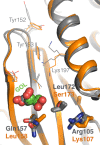




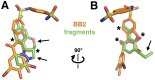




References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Andersen HS, Olsen OH, Iversen LF, Sørensen AL, Mortensen SB, Christensen MS, Branner S, Hansen TK, Lau JF, Jeppesen L, Moran EJ, Su J, Bakir F, Judge L, Shahbaz M, Collins T, Vo T, Newman MJ, Ripka WC, Møller NP. Discovery and SAR of a novel selective and orally bioavailable nonpeptide classical competitive inhibitor class of protein-tyrosine phosphatase 1B. Journal of Medicinal Chemistry. 2002;45:4443–4459. doi: 10.1021/jm0209026. - DOI - PubMed
-
- Bandyopadhyay D, Kusari A, Kenner KA, Liu F, Chernoff J, Gustafson TA, Kusari J. Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. Journal of Biological Chemistry. 1997;272:1639–1645. doi: 10.1074/jbc.272.3.1639. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 CA203002/CA/NCI NIH HHS/United States
- CA191018/CA/NCI NIH HHS/United States
- P30 GM124169/GM/NIGMS NIH HHS/United States
- GM124169/GM/NIGMS NIH HHS/United States
- GM123159/GM/NIGMS NIH HHS/United States
- GM110580/GM/NIGMS NIH HHS/United States
- K99CA203002/CA/NCI NIH HHS/United States
- R01 GM123159/GM/NIGMS NIH HHS/United States
- T32 GM064337/GM/NIGMS NIH HHS/United States
- Wellcome Trust/United Kingdom
- F31 CA180378/CA/NCI NIH HHS/United States
- (F31 CA180378/CA/NCI NIH HHS/United States
- GM124149/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

