Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated
- PMID: 29878075
- PMCID: PMC6022546
- DOI: 10.1093/brain/awy146
Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated
Abstract
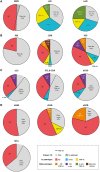
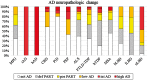
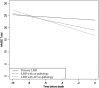
Lewy bodies commonly occur in Alzheimer's disease, and Alzheimer's disease pathology is frequent in Lewy body diseases, but the burden of co-pathologies across neurodegenerative diseases is unknown. We assessed the extent of tau, amyloid-β, α-synuclein and TDP-43 proteinopathies in 766 autopsied individuals representing a broad spectrum of clinical neurodegenerative disease. We interrogated pathological Alzheimer's disease (n = 247); other tauopathies (n = 95) including Pick's disease, corticobasal disease and progressive supranuclear palsy; the synucleinopathies (n = 164) including multiple system atrophy and Lewy body disease; the TDP-43 proteinopathies (n = 188) including frontotemporal lobar degeneration with TDP-43 inclusions and amyotrophic lateral sclerosis; and a minimal pathology group (n = 72). Each group was divided into subgroups without or with co-pathologies. Age and sex matched logistic regression models compared co-pathology prevalence between groups. Co-pathology prevalence was similar between the minimal pathology group and most neurodegenerative diseases for each proteinopathy: tau was nearly universal (92-100%), amyloid-β common (20-57%); α-synuclein less common (4-16%); and TDP-43 the rarest (0-16%). In several neurodegenerative diseases, co-pathology increased: in Alzheimer's disease, α-synuclein (41-55%) and TDP-43 (33-40%) increased; in progressive supranuclear palsy, α-synuclein increased (22%); in corticobasal disease, TDP-43 increased (24%); and in neocortical Lewy body disease, amyloid-β (80%) and TDP-43 (22%) increased. Total co-pathology prevalence varied across groups (27-68%), and was increased in high Alzheimer's disease, progressive supranuclear palsy, and neocortical Lewy body disease (70-81%). Increased age at death was observed in the minimal pathology group, amyotrophic lateral sclerosis, and multiple system atrophy cases with co-pathologies. In amyotrophic lateral sclerosis and neocortical Lewy body disease, co-pathologies associated with APOE ɛ4. Lewy body disease cases with Alzheimer's disease co-pathology had substantially lower Mini-Mental State Examination scores than pure Lewy body disease. Our data imply that increased age and APOE ɛ4 status are risk factors for co-pathologies independent of neurodegenerative disease; that neurodegenerative disease severity influences co-pathology as evidenced by the prevalence of co-pathology in high Alzheimer's disease and neocortical Lewy body disease, but not intermediate Alzheimer's disease or limbic Lewy body disease; and that tau and α-synuclein strains may also modify co-pathologies since tauopathies and synucleinopathies had differing co-pathologies and burdens. These findings have implications for clinical trials that focus on monotherapies targeting tau, amyloid-β, α-synuclein and TDP-43.
Figures

Comment in
-
A broader view of dementia: multiple co-pathologies are the norm.Brain. 2018 Jul 1;141(7):1894-1897. doi: 10.1093/brain/awy153. Brain. 2018. PMID: 30053176 No abstract available.
References
-
- Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol 2001; 54: 343–9. - PubMed
-
- Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 2015; 129: 221–37. - PMC - PubMed
-
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011; 70: 960–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

