Emergent Functional Network Effects in Parkinson Disease
- PMID: 29878081
- PMCID: PMC6519699
- DOI: 10.1093/cercor/bhy121
Emergent Functional Network Effects in Parkinson Disease
Erratum in
-
Emergent Functional Network Effects in Parkinson Disease.Cereb Cortex. 2019 Apr 1;29(4):1701. doi: 10.1093/cercor/bhy229. Cereb Cortex. 2019. PMID: 30418543 Free PMC article. No abstract available.
Abstract
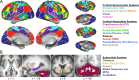
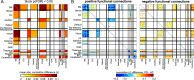
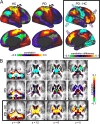
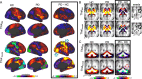
The hallmark pathology underlying Parkinson disease (PD) is progressive synucleinopathy, beginning in caudal brainstem that later spreads rostrally. However, the primarily subcortical pathology fails to account for the wide spectrum of clinical manifestations in PD. To reconcile these observations, resting-state functional connectivity (FC) can be used to examine dysfunction across distributed brain networks. We measured FC in a large, single-site study of nondemented PD (N = 107; OFF medications) and healthy controls (N = 46) incorporating rigorous quality control measures and comprehensive sampling of cortical, subcortical and cerebellar regions. We employed novel statistical approaches to determine group differences across the entire connectome, at the network-level, and for select brain regions. Group differences respected well-characterized network delineations producing a striking "block-wise" pattern of network-to-network effects. Surprisingly, these results demonstrate that the greatest FC differences involve sensorimotor, thalamic, and cerebellar networks, with notably smaller striatal effects. Split-half replication demonstrates the robustness of these results. Finally, block-wise FC correlations with behavior suggest that FC disruptions may contribute to clinical manifestations in PD. Overall, these results indicate a concerted breakdown of functional network interactions, remote from primary pathophysiology, and suggest that FC deficits in PD are related to emergent network-level phenomena rather than focal pathology.
Keywords: Parkinson disease; fMRI; functional connectivity; networks.
© The Author(s) 2018. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Aarsland D, Kramberger MG. 2015. Neuropsychiatric symptoms in Parkinson’s disease. J Parkinsons Dis. 5:659–667. - PubMed
-
- Abbruzzese G, Berardelli A. 2003. Sensorimotor integration in movement disorders. Mov Disord. 18:231–240. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. 1990. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 85:119–146. - PubMed
-
- Amboni M, Tessitore A, Esposito F, Santangelo G, Picillo M, Vitale C, Giordano A, Erro R, de Micco R, Corbo D, et al. . 2015. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J Neurol. 262:425–434. - PubMed
-
- Astafiev SV, Snyder AZ, Shulman GL, Corbetta M. 2010. Comment on “Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI” and “Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area”. Science. 328:309; author reply 309. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

