Adjuvant Anti-HER2 Therapy, Treatment-Related Amenorrhea, and Survival in Premenopausal HER2-Positive Early Breast Cancer Patients
- PMID: 29878225
- PMCID: PMC6335113
- DOI: 10.1093/jnci/djy094
Adjuvant Anti-HER2 Therapy, Treatment-Related Amenorrhea, and Survival in Premenopausal HER2-Positive Early Breast Cancer Patients
Erratum in
-
Corrigendum.J Natl Cancer Inst. 2019 Nov 1;111(11):1237. doi: 10.1093/jnci/djy197. J Natl Cancer Inst. 2019. PMID: 30329078 Free PMC article. No abstract available.
Abstract
Background: In premenopausal patients with human epidermal growth factor receptor 2 (HER2)-positive early breast cancer, the gonadotoxicity of trastuzumab and lapatinib remains largely uncertain, and the prognostic effect of treatment-related amenorrhea (TRA) is unknown.
Methods: In the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (BIG 2-06) phase III trial, HER2-positive early breast cancer patients were randomized (1:1:1:1) to receive one year of trastuzumab, lapatinib, their sequence, or their combination. As per study protocol, menopausal status was collected in all patients at random assignment and at week 37 visit. We investigated TRA rates and whether TRA in patients with hormone receptor-positive and -negative tumors would impact disease-free survival (DFS) and overall survival (OS). Landmark and time-dependent modeling were used to account for guarantee-time bias. All statistical tests were two-sided.
Results: A total of 2862 premenopausal women were included, of whom 1679 (58.7%) had hormone receptor-positive disease. Median age was 43 (interquartile range = 38-47) years. Similar TRA rates were observed in the trastuzumab (72.6%), lapatinib (74.0%), trastuzumab→lapatinib (72.1%), and trastuzumab+lapatinib (74.8%) arms (P = .64). The association between TRA and survival outcomes differed according to hormone-receptor status (Pinteraction for DFS = .007; Pinteraction for OS = .003). For hormone receptor-positive patients, the TRA cohort had statistically significantly better DFS (adjusted hazard ratio [aHR] = 0.58, 95% confidence interval [CI] = 0.45 to 0.76) and OS (aHR = 0.63, 95% CI = 0.40 to 0.99) than the no TRA cohort. No difference was observed in hormone receptor-negative patients.
Conclusions: In this unplanned analysis, no association between TRA rate and type of anti-HER2 treatment was observed. TRA was associated with statistically significant survival benefits in premenopausal hormone receptor-positive/HER2-positive early breast cancer patients.
Figures
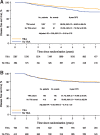

Comment in
-
What Can We Learn From Menstrual Patterns After Treatment for HER2-Positive Breast Cancer?J Natl Cancer Inst. 2019 Jan 1;111(1):9-10. doi: 10.1093/jnci/djy092. J Natl Cancer Inst. 2019. PMID: 29878141 Free PMC article. No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A.. Cancer Statistics, 2018. CA Cancer J Clin. 2018;681:7–30. - PubMed
-
- Denduluri N, Somerfield MR, Eisen A, et al. Selection of optimal adjuvant chemotherapy regimens for human epidermal growth factor receptor 2 (HER2)-negative and adjuvant targeted therapy for HER2-positive breast cancers: An American Society of Clinical Oncology guideline adaptation of the Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol. 2016;3420:2416–2427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

