Chronic myelomonocytic leukemia: 2018 update on diagnosis, risk stratification and management
- PMID: 29878489
- PMCID: PMC5995129
- DOI: 10.1002/ajh.25104
Chronic myelomonocytic leukemia: 2018 update on diagnosis, risk stratification and management
Abstract
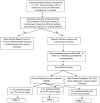
Disease overview: Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic stem cell disorder with overlapping features of myelodysplastic syndromes and myeloproliferative neoplasms, with an inherent risk for leukemic transformation (∼15%-20% over 3-5 years).
Diagnosis: Diagnosis is based on the presence of sustained (>3 months) peripheral blood monocytosis (≥1 × 109 /L; monocytes ≥10%), along with bone marrow dysplasia. Clonal cytogenetic abnormalities occur in ∼ 30% of patients, while >90% have gene mutations. Mutations involving TET2 (∼60%), SRSF2 (∼50%), ASXL1 (∼40%) and the oncogenic RAS pathway (∼30%) are frequent; while the presence of ASXL1 and DNMT3A mutations and the absence of TET2 mutations negatively impact over-all survival.
Risk stratification: Molecularly integrated prognostic models include; the Groupe Français des Myélodysplasies (GFM), Mayo Molecular Model (MMM), and the CMML specific prognostic model (CPSS-Mol). Risk factors incorporated into the MMM include presence of nonsense or frameshift ASXL1 mutations, absolute monocyte count > 10 × 109 /L, hemoglobin <10 gm/dL, platelet count <100 × 109 /L and the presence of circulating immature myeloid cells. The MMM stratifies CMML patients into 4 groups; high (≥3 risk factors), intermediate-2 (2 risk factors), intermediate-1 (1 risk factor), and low (no risk factors), with median survivals of 16, 31, 59, and 97 months, respectively.
Risk-adapted therapy: Hypomethylating agents such as 5-azacitidine and decitabine are commonly used, with overall response rates of ∼30%-40% and complete remission rates of ∼7%-17%; with no impact on mutational allele burdens. Allogeneic stem cell transplant is the only potentially curative option, but is associated with significant morbidity and mortality.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures


References
-
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. - PubMed
-
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. - PubMed
-
- Ricci C, Fermo E, Corti S, et al. RAS mutations contribute to evolution of chronic myelomonocytic leukemia to the proliferative variant. Clin Cancer Res. 2010;16:2246–2256. - PubMed
-
- Ades L, Sekeres MA, Wolfromm A, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013;37:609–613. - PubMed
-
- Patnaik MM, Lasho TL, Finke CM, et al. Spliceosome mutations involving SRSF2, SF3B1, and U2AF35 in chronic myelomonocytic leukemia: prevalence, clinical correlates, and prognostic relevance. Am J Hematol. 2013;88:201–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
