Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial
- PMID: 29878866
- PMCID: PMC6366953
- DOI: 10.1200/JCO.2018.78.5865
Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial
Abstract
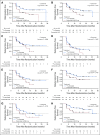
Purpose Neuroendocrine tumor (NET) progression is associated with deterioration in quality of life (QoL). We assessed the impact of 177Lu-Dotatate treatment on time to deterioration in health-related QoL. Methods The NETTER-1 trial is an international phase III study in patients with midgut NETs. Patients were randomly assigned to treatment with 177Lu-Dotatate versus high-dose octreotide. European Organisation for Research and Treatment of Cancer quality-of-life questionnaires QLQ C-30 and G.I.NET-21 were assessed during the trial to determine the impact of treatment on health-related QoL. Patients completed the questionnaires at baseline and every 12 weeks until tumor progression. QoL scores were converted to a 100-point scale according to European Organisation for Research and Treatment of Cancer instructions, and individual changes from baseline scores were assessed. Time to QoL deterioration (TTD) was defined as the time from random assignment to the first QoL deterioration ≥ 10 points for each patient in the corresponding domain scale. All analyses were conducted on the intention-to-treat population. Patients with no deterioration were censored at the last QoL assessment date. Results TTD was significantly longer in the 177Lu-Dotatate arm (n = 117) versus the control arm (n = 114) for the following domains: global health status (hazard ratio [HR], 0.406), physical functioning (HR, 0.518), role functioning (HR, 0.580), fatigue (HR, 0.621), pain (HR, 0.566), diarrhea (HR, 0.473), disease-related worries (HR, 0.572), and body image (HR, 0.425). Differences in median TTD were clinically significant in several domains: 28.8 months versus 6.1 months for global health status, and 25.2 months versus 11.5 months for physical functioning. Conclusion This analysis from the NETTER-1 phase III study demonstrates that, in addition to improving progression-free survival, 177Lu-Dotatate provides a significant QoL benefit for patients with progressive midgut NETs compared with high-dose octreotide.
Trial registration: ClinicalTrials.gov NCT01578239.
Figures

References
-
- Kulke MH, Benson AB, III, Bergsland E, et al. : Neuroendocrine tumors. J Natl Compr Canc Netw 10:724-764, 2012 - PubMed
-
- Rinke A, Müller HH, Schade-Brittinger C, et al. : Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol 27:4656-4663, 2009 - PubMed
-
- Caplin ME, Pavel M, Ćwikła JB, et al. : Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371:224-233, 2014 - PubMed
-
- Beaumont JL, Cella D, Phan AT, et al. : Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 41:461-466, 2012 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

