Chimeric antigen receptor T-cell approaches to HIV cure
- PMID: 29878913
- PMCID: PMC6993924
- DOI: 10.1097/COH.0000000000000485
Chimeric antigen receptor T-cell approaches to HIV cure
Abstract
Purpose of review: Combination antiretroviral therapy (ART) has enabled tremendous progress in suppressing HIV replication in infected patients. However, ART alone cannot eradicate HIV and its latent, persisting reservoirs. Novel approaches are needed to eradicate the virus or achieve functional cure in the absence of ART.
Recent findings: Adoptive T-cell therapies were initially tested in HIV-infected individuals with limited efficiency. Benefiting from new and improved methodologies, an increasing array of CAR T-cell therapies has been successfully developed in the cancer immunotherapy field, demonstrating promising new avenues that could be applied to HIV. Numerous studies have characterized various HIV-specific CAR constructs, types of cytolytic effector cells, and CAR-expressing cells' trafficking to the reservoir compartments, warranting further in-vivo efforts. Notably, the ability of CAR cells to persist and function in low-antigen environments in vivo, that is, in ART-suppressed patients, remains unclear.
Summary: Despite promising results in preclinical studies, only a handful of clinical trials have been initiated worldwide. Several obstacles remain prior to successful application of HIV-specific CAR T-cell therapies in patients. In this review, we survey the current state of the field, and address paths towards realizing the goal of an efficacious HIV CAR T-cell product.
Conflict of interest statement
Conflicts of interest
None.
Figures
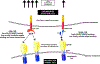
References
-
- Borrow P, Lewicki H, Wei X, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med 1997; 3(2):205–211. - PubMed
-
- Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell 1991; 64(5):1037–1046. - PubMed
-
- Roberts MR, Qin L, Zhang D, et al. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood 1994; 84(9):2878–2889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

