Extracellular mRNA detected by molecular beacons in tethered lipoplex nanoparticles for diagnosis of human hepatocellular carcinoma
- PMID: 29879168
- PMCID: PMC5991670
- DOI: 10.1371/journal.pone.0198552
Extracellular mRNA detected by molecular beacons in tethered lipoplex nanoparticles for diagnosis of human hepatocellular carcinoma
Abstract
Hepatocellular carcinoma (HCC) remains one of the major causes of cancer related deaths. Although ultrasonography (US), computed tomography (CT) and/or high-cost magnetic resonance imaging (MRI) have been shown to improve early detection of liver cancer and mortality rates in high-risk individuals, such imaging based methods are limited by high rates of false positivity leading to unnecessary patient anxiety and invasive procedures. Complementary blood biomarkers could increase the accuracy of early detection. Although Alpha-fetoprotein (AFP) in blood is widely used in HCC screening and diagnosis, the false-negative rate as high as 30% and 40% is found in advanced HCC and early stage HCC respectively. We detected AFP messenger RNA (mRNA) in extracellular vesicles (EVs) in patient plasma using designed molecular beacons and a novel tethered lipoplex nanoparticle (TLN) biochip. Together with glypican-3 (GPC-3) mRNA, another well-known HCC marker, we observed much improved performance of AFP protein-based HCC detection. Comparing normal donors (N = 38) and HCC patients (N = 40), our TLN biochip using EV AFP and GPC-3 mRNAs provided an AUC (area under the ROC curve) of 0.995, better than that of a single marker. This 2-mRNA combination also provided a perfect positive predictive value (PPV = 1) at a negative predictive value (NPV) of 0.95 and 20% prevalence, while the blood AFP protein or plasma EV GPC3 mRNA alone could only provide a PPV of 0.61 and 0.79 respectively at the same conditions. Thus, this facile new method may complement current models for risk stratification in liver cancer screening, therapeutic monitoring, and after-treatment surveillance. However, large scale validation will need to be conducted to confirm its clinical potential.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

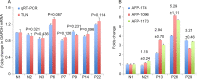
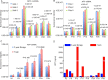

References
-
- Li R, Yang D, Tang CL, Cai P, Ma KS, Ding SY, et al. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer. 2016;16:158 doi: 10.1186/s12885-016-2156-x - DOI - PMC - PubMed
-
- Yao M, Yao DF, Bian YZ, Wu W, Yan XD, Yu DD, Qiu LW, et al. Values of circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:171–179. - PubMed
-
- Shirakawa H, Kuronuma T, Nishimura Y, Hasebe T, Nakano M, Gotohda N, et al. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. Int J Oncol. 2009; 34:649–656. - PubMed
-
- Gao W, Tang Z, Zhang YF, Feng M, Qian M, Dimitrov DS, et al. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun. 2015;6:6536 doi: 10.1038/ncomms7536 - DOI - PMC - PubMed
-
- Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J; et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

