MRAP deficiency impairs adrenal progenitor cell differentiation and gland zonation
- PMID: 29879378
- PMCID: PMC6181639
- DOI: 10.1096/fj.201701274RR
MRAP deficiency impairs adrenal progenitor cell differentiation and gland zonation
Abstract
Melanocortin 2 receptor accessory protein (MRAP) is a single transmembrane domain accessory protein and a critical component of the hypothamo-pituitary-adrenal axis. MRAP is highly expressed in the adrenal gland and is essential for adrenocorticotropin hormone (ACTH) receptor expression and function. Human loss-of-function mutations in MRAP cause familial glucocorticoid (GC) deficiency (FGD) type 2 (FGD2), whereby the adrenal gland fails to respond to ACTH and to produce cortisol. In this study, we generated Mrap-null mice to study the function of MRAP in vivo. We found that the vast majority of Mrap-/- mice died at birth but could be rescued by administration of corticosterone to pregnant dams. Surviving Mrap-/- mice developed isolated GC deficiency with normal mineralocorticoid and catecholamine production, recapitulating FGD2. The adrenal glands of adult Mrap-/- mice were small, with grossly impaired adrenal capsular morphology and cortex zonation. Progenitor cell differentiation was significantly impaired, with dysregulation of WNT4/β-catenin and sonic hedgehog pathways. These data demonstrate the roles of MRAP in both steroidogenesis and the regulation of adrenal cortex zonation. This is the first mouse model of isolated GC deficiency and reveals the role of MRAP in adrenal progenitor cell regulation and cortex zonation.-Novoselova, T. V., Hussain, M., King, P. J., Guasti, L., Metherell, L. A., Charalambous, M., Clark, A. J. L., Chan, L. F. MRAP deficiency impairs adrenal progenitor cell differentiation and gland zonation.
Keywords: ACTH; accessory protein; cell fate; familial glucocorticoid deficiency; melanocortin.
Conflict of interest statement
This work was supported by The Medical Research Council (MRC) UK/Academy of Medical Sciences Clinician Scientist Fellowship Grant G0802796 (to L.F.C., supporting T.V.N.), a Society for Endocrinology Early Career award (to T.V.N.), MRC Collaborative Awards in Science and Engineering (CASE) Studentship Grant MR/J006394/1 (to A.J.L.C. and L.F.C. supporting M.H.), Biotechnology and Biological Sciences Research Council Award BB/L00267/1 and funds from the Rosetrees Trust (to L.G.), MRC Grant UK MR/K020455/1 (to L.M.), and MRC Grant MR/L002345/1 (to M.C.). The authors thank Dr. C. E. Gomez-Sanches (University of Mississippi, Jackson, MI, USA) for the kind gift of CYP11B1 and CYP11B2 antibody, Dr. Ed Laufer (Columbia University, NY, USA) for technical advice, Prof. Yacob Weinstein (Ben-Gurion University of the Negev, Israel) for the 20-αHSD antibody, Anthony Price and the staff at the Biological Services Unit at Queen Mary University of London for technical assistance, Dr. Anthony Coll and Sir/Prof. Steven O’Rahilly (Institute of Metabolic Sciences, Cambridge, Uninted Kingdom) for help with conceptualization and support of the project, Keith Burling and Peter Barker [Cambridge University Hospitals National Health Service (NHS) Foundation Trust] for the provision of laboratory services, and David Jackson (QMUL) for the critical reading of the manuscript. The authors declare no conflicts of interest.
Figures

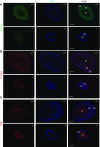

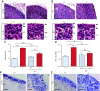
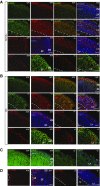
References
-
- Metherell L. A., Chapple J. P., Cooray S., David A., Becker C., Rüschendorf F., Naville D., Begeot M., Khoo B., Nürnberg P., Huebner A., Cheetham M. E., Clark A. J. (2005) Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet. 37, 166–170 - PubMed
-
- Chan L. F., Webb T. R., Chung T. T., Meimaridou E., Cooray S. N., Guasti L., Chapple J. P., Egertová M., Elphick M. R., Cheetham M. E., Metherell L. A., Clark A. J. (2009) MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc. Natl. Acad. Sci. USA 106, 6146–6151 - PMC - PubMed
-
- Novoselova T. V., Jackson D., Campbell D. C., Clark A. J., Chan L. F. (2013) Melanocortin receptor accessory proteins in adrenal gland physiology and beyond. J. Endocrinol. 217, R1–R11 - PubMed
-
- Asai M., Ramachandrappa S., Joachim M., Shen Y., Zhang R., Nuthalapati N., Ramanathan V., Strochlic D. E., Ferket P., Linhart K., Ho C., Novoselova T. V., Garg S., Ridderstråle M., Marcus C., Hirschhorn J. N., Keogh J. M., O’Rahilly S., Chan L. F., Clark A. J., Farooqi I. S., Majzoub J. A. (2013) Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341, 275–278 - PMC - PubMed
-
- Novoselova T. V., Larder R., Rimmington D., Lelliott C., Wynn E. H., Gorrigan R. J., Tate P. H., Guasti L., O’Rahilly S., Clark A. J., Logan D. W., Coll A. P., Chan L. F.; Sanger Mouse Genetics Project (2016) Loss of Mrap2 is associated with Sim1 deficiency and increased circulating cholesterol. J. Endocrinol. 230, 13–26 - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

