Secukinumab provides rapid and sustained pain relief in psoriatic arthritis over 2 years: results from the FUTURE 2 study
- PMID: 29880010
- PMCID: PMC5992664
- DOI: 10.1186/s13075-018-1610-3
Secukinumab provides rapid and sustained pain relief in psoriatic arthritis over 2 years: results from the FUTURE 2 study
Abstract
Background: Pain is one of the most important domains affecting health-related quality of life (HRQoL) in patients with psoriatic arthritis (PsA). Secukinumab has demonstrated rapid and sustained improvements in signs and symptoms, including HRQoL, among patients with active PsA. This analysis evaluates the effect of secukinumab on patient-reported pain in PsA through 104 weeks of treatment.
Methods: Pain was assessed through week 104 using clinically relevant measures, including change from baseline in a pain visual analog scale (VAS) and Short Form-36 (SF-36) bodily domain scores; proportion of patients reporting improvements equal to or better than minimum clinically meaningful differences in the pain VAS and SF-36 bodily pain domain scores; and proportion of patients with no, moderate, or extreme pain/discomfort measured by the EuroQoL 5-Dimension 3-Level Questionnaire (EQ-5D-3 L) pain item scores. Correlations of pain measures were analyzed using Pearson's correlation coefficient. Pre-specified analyses of TNF-naïve patients and patients who stopped TNF-inhibitors (TNFis) due to inadequate responses or safety/tolerability (TNF-IR patients) were performed using "as-observed data."
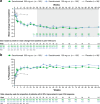
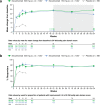
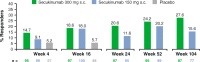
Results: Mean improvements from baseline in pain VAS scores were greater with secukinumab versus placebo by week 3 (- 16.9; P < 0.0001 with secukinumab 300 mg and - 12.6; P < 0.05 with secukinumab 150 mg) and sustained through week 104. SF-36 bodily pain domain scores were significantly greater with 300 mg secukinumab and secukinumab 150 mg versus placebo by week 4 (16.2 and 16.3, respectively; P < 0.0001 for both), and these changes were maintained through week 104. With both secukinumab 300 mg and secukinumab 150 mg, improvements equal to or better than the minimum clinically meaningful differences in pain VAS and SF-36 bodily pain were significant versus placebo at week 3 and week 4, respectively. At week 4, 15%, 9%, and 5% of patients receiving secukinumab 300 mg, secukinumab 150 mg, and placebo, respectively, reported "no pain/discomfort" measured by EQ-5D-3 L; these proportions increased to week 104 with both secukinumab doses. Similarly, improvements in pain measures were significant in both TNF-naïve and TNF-IR patients.
Conclusion: Secukinumab provided rapid and sustained pain relief in PsA over 2 years of treatment. Improvements in pain were reported regardless of prior exposure to TNFis.
Trial registration: ClinicalTrials.gov, NCT01752634 . Registered on 19 December 2012.
Keywords: Pain relief; Psoriatic arthritis; Secukinumab.
Conflict of interest statement
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki, and all centers received approval from independent ethics committees or institutional review boards.
Competing interests
IB McInnes received research grants, consultation fees, or speaker honoraria from AbbVie, Amgen, BMS, Celgene, Janssen, Lilly, Novartis, Pfizer, and UCB. PJ Mease received research grants, consultation fees, or speaker honoraria from AbbVie, Amgen, BMS, Celgene, Crescendo Bioscience, Genentech, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB. G Schett received speaker fees from Abbvie, BMS, Celgene, Chugai, Eli Lilly, Janssen, Novartis, Roche, and UCB. B Kirkham received research support from AbbVie, Novartis, Roche; served as a consultant and speaker for Abbott, BMS, Chugai, MSD, Novartis, Pfizer, Roche, UCB; and participated in speakers bureaus for Abbott, BMS, Chugai, MSD, Novartis, Pfizer, Roche, UCB. V Strand is a founding member of the executive of Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT), an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies; a member of the American College of Rheumatology’s Clinical Trials Task Force and Chair of the American College of Rheumatology/OMERACT Imaging Subcommittee. Dr. Strand also has served as a consultant and member of advisory boards for AbbVie, Alder, Amgen Corporation, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celltrion, Genentech/Roche, GlaxoSmithKline, Janssen, Eli Lily, Novartis, Pfizer, Regeneron, Sandoz, Sanofi, and UCB. N Williams is a consultant for Novartis through employment at RTI Health Solutions and is an employee of RTI Health Solutions. T Fox, L Pricop, SM Jugl, and KK Gandhi are employees of Novartis and own Novartis stock.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3).Arthritis Res Ther. 2018 Mar 15;20(1):47. doi: 10.1186/s13075-018-1551-x. Arthritis Res Ther. 2018. PMID: 29544534 Free PMC article. Clinical Trial.
-
Secukinumab provides sustained PASDAS-defined remission in psoriatic arthritis and improves health-related quality of life in patients achieving remission: 2-year results from the phase III FUTURE 2 study.Arthritis Res Ther. 2018 Dec 7;20(1):272. doi: 10.1186/s13075-018-1773-y. Arthritis Res Ther. 2018. PMID: 30526678 Free PMC article. Clinical Trial.
-
Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study.Rheumatology (Oxford). 2017 Nov 1;56(11):1993-2003. doi: 10.1093/rheumatology/kex301. Rheumatology (Oxford). 2017. PMID: 28968735 Free PMC article. Clinical Trial.
-
Secukinumab: A Review in Psoriatic Arthritis.Drugs. 2016 Jul;76(11):1135-45. doi: 10.1007/s40265-016-0602-3. Drugs. 2016. PMID: 27299434 Review.
-
Secukinumab: A New Treatment Option for Psoriatic Arthritis.Rheumatol Ther. 2016 Jun;3(1):5-29. doi: 10.1007/s40744-016-0031-5. Epub 2016 Apr 23. Rheumatol Ther. 2016. PMID: 27747518 Free PMC article. Review.
Cited by
-
Psoriatic arthritis from a mechanistic perspective.Nat Rev Rheumatol. 2022 Jun;18(6):311-325. doi: 10.1038/s41584-022-00776-6. Epub 2022 May 5. Nat Rev Rheumatol. 2022. PMID: 35513599 Review.
-
A Matching-Adjusted Indirect Comparison of Guselkumab and Secukinumab in Patients with Psoriatic Arthritis Over 52 Weeks.Rheumatol Ther. 2025 Aug;12(4):663-677. doi: 10.1007/s40744-025-00771-9. Epub 2025 Jun 1. Rheumatol Ther. 2025. PMID: 40450642 Free PMC article.
-
Varicella Zoster and Cutaneous Candida Infection in a Patient With Ankylosing Spondylitis Under Treatment With Secukinumab.Arch Rheumatol. 2019 Nov 6;35(1):151-153. doi: 10.5606/ArchRheumatol.2020.7545. eCollection 2020 Mar. Arch Rheumatol. 2019. PMID: 32637934 Free PMC article. No abstract available.
-
Effect of upadacitinib on reducing pain in patients with active psoriatic arthritis or ankylosing spondylitis: post hoc analysis of three randomised clinical trials.RMD Open. 2022 Mar;8(1):e002049. doi: 10.1136/rmdopen-2021-002049. RMD Open. 2022. PMID: 35332058 Free PMC article. Clinical Trial.
-
Secukinumab: A Review in Psoriatic Arthritis.Drugs. 2021 Mar;81(4):483-494. doi: 10.1007/s40265-021-01476-3. Epub 2021 Mar 4. Drugs. 2021. PMID: 33661486 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

