Targeting tumour re-wiring by triple blockade of mTORC1, epidermal growth factor, and oestrogen receptor signalling pathways in endocrine-resistant breast cancer
- PMID: 29880014
- PMCID: PMC5992820
- DOI: 10.1186/s13058-018-0983-1
Targeting tumour re-wiring by triple blockade of mTORC1, epidermal growth factor, and oestrogen receptor signalling pathways in endocrine-resistant breast cancer
Erratum in
-
Correction: Targeting tumour re-wiring by triple blockade of mTORC1, epidermal growth factor, and oestrogen receptor signalling pathways in endocrine-resistant breast cancer.Breast Cancer Res. 2025 Jun 24;27(1):116. doi: 10.1186/s13058-025-02046-1. Breast Cancer Res. 2025. PMID: 40556031 Free PMC article. No abstract available.
Abstract
Background: Endocrine therapies are the mainstay of treatment for oestrogen receptor (ER)-positive (ER+) breast cancer (BC). However, resistance remains problematic largely due to enhanced cross-talk between ER and growth factor pathways, circumventing the need for steroid hormones. Previously, we reported the anti-proliferative effect of everolimus (RAD001-mTORC1 inhibitor) with endocrine therapy in resistance models; however, potential routes of escape from treatment via ERBB2/3 signalling were observed. We hypothesised that combined targeting of three cellular nodes (ER, ERBB, and mTORC1) may provide enhanced long-term clinical utility.
Methods: A panel of ER+ BC cell lines adapted to long-term oestrogen deprivation (LTED) and expressing ESR1 wt or ESR1 Y537S , modelling acquired resistance to an aromatase-inhibitor (AI), were treated in vitro with a combination of RAD001 and neratinib (pan-ERBB inhibitor) in the presence or absence of oestradiol (E2), tamoxifen (4-OHT), or fulvestrant (ICI182780). End points included proliferation, cell signalling, cell cycle, and effect on ER-mediated transactivation. An in-vivo model of AI resistance was treated with monotherapies and combinations to assess the efficacy in delaying tumour progression. RNA-seq analysis was performed to identify changes in global gene expression as a result of the indicated therapies.
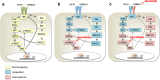
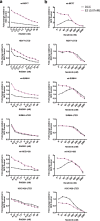
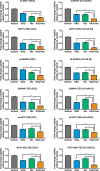
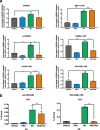
Results: Here, we show RAD001 and neratinib (pan-ERBB inhibitor) caused a concentration-dependent decrease in proliferation, irrespective of the ESR1 mutation status. The combination of either agent with endocrine therapy further reduced proliferation but the maximum effect was observed with a triple combination of RAD001, neratinib, and endocrine therapy. In the absence of oestrogen, RAD001 caused a reduction in ER-mediated transcription in the majority of the cell lines, which associated with a decrease in recruitment of ER to an oestrogen-response element on the TFF1 promoter. Contrastingly, neratinib increased both ER-mediated transactivation and ER recruitment, an effect reduced by the addition of RAD001. In-vivo analysis of an LTED model showed the triple combination of RAD001, neratinib, and fulvestrant was most effective at reducing tumour volume. Gene set enrichment analysis revealed that the addition of neratinib negated the epidermal growth factor (EGF)/EGF receptor feedback loops associated with RAD001.
Conclusions: Our data support the combination of therapies targeting ERBB2/3 and mTORC1 signalling, together with fulvestrant, in patients who relapse on endocrine therapy and retain a functional ER.
Keywords: Breast cancer; Endocrine resistance; Everomilus; Neratinib; Oestrogen receptor.
Conflict of interest statement
Ethics approval and consent to participate
In-vivo studies were carried out in accordance with Home Office guidelines and approved by the Institute of Cancer Research Ethics Committee.
Consent for publication
All authors approved the final version of this manuscript.
Competing interests
L-AM and SRJ receive academic funding from PUMA Biotechnology. FA-C, ASL, and REC are employees of PUMA Biotechnology. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women.Cochrane Database Syst Rev. 2009 Oct 7;2009(4):CD004562. doi: 10.1002/14651858.CD004562.pub4. Cochrane Database Syst Rev. 2009. PMID: 19821328 Free PMC article.
-
FGFR4 in endocrine resistance: overexpression and estrogen regulation without direct causative role.Breast Cancer Res Treat. 2025 Jun;211(2):501-515. doi: 10.1007/s10549-025-07666-x. Epub 2025 Mar 17. Breast Cancer Res Treat. 2025. PMID: 40097769
-
Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003370. doi: 10.1002/14651858.CD003370.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. PMID: 17253488 Updated.
-
PARP-1 as a novel target in endocrine-resistant breast cancer.J Exp Clin Cancer Res. 2025 Jun 16;44(1):175. doi: 10.1186/s13046-025-03441-4. J Exp Clin Cancer Res. 2025. PMID: 40518539 Free PMC article.
-
Mammographic density, endocrine therapy and breast cancer risk: a prognostic and predictive biomarker review.Cochrane Database Syst Rev. 2021 Oct 26;10(10):CD013091. doi: 10.1002/14651858.CD013091.pub2. Cochrane Database Syst Rev. 2021. PMID: 34697802 Free PMC article.
Cited by
-
Association of functional genetic variants in TFF1 and nephrolithiasis risk in a Chinese population.BMC Urol. 2022 Aug 20;22(1):127. doi: 10.1186/s12894-022-01081-w. BMC Urol. 2022. PMID: 35987613 Free PMC article.
-
Estrogen/HER2 receptor crosstalk in breast cancer: combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer.NPJ Breast Cancer. 2023 May 31;9(1):45. doi: 10.1038/s41523-023-00533-2. NPJ Breast Cancer. 2023. PMID: 37258523 Free PMC article. Review.
-
Endocrine Resistance in Hormone Receptor Positive Breast Cancer-From Mechanism to Therapy.Front Endocrinol (Lausanne). 2019 May 24;10:245. doi: 10.3389/fendo.2019.00245. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31178825 Free PMC article. Review.
-
Regulation of aromatase in cancer.Mol Cell Biochem. 2021 Jun;476(6):2449-2464. doi: 10.1007/s11010-021-04099-0. Epub 2021 Feb 18. Mol Cell Biochem. 2021. PMID: 33599895 Review.
-
Advances in the Detection Technologies and Clinical Applications of Circulating Tumor DNA in Metastatic Breast Cancer.Cancer Manag Res. 2020 May 18;12:3547-3560. doi: 10.2147/CMAR.S249041. eCollection 2020. Cancer Manag Res. 2020. PMID: 32547192 Free PMC article. Review.
References
-
- Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys. 2015;72(2):333–8. - PubMed
-
- Drury SC, Detre S, Leary A, Salter J, Reis-Filho J, Barbashina V, Marchio C, Lopez-Knowles E, Ghazoui Z, Habben K, et al. Changes in breast cancer biomarkers in the IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen treatment. Endocr Relat Cancer. 2011;18(5):565–577. doi: 10.1530/ERC-10-0046. - DOI - PubMed
-
- Arnedos M, Drury S, Afentakis M, A'Hern R, Hills M, Salter J, Smith IE, Reis-Filho JS, Dowsett M. Biomarker changes associated with the development of resistance to aromatase inhibitors (AIs) in estrogen receptor-positive breast cancer. Ann Oncol. 2014;25(3):605–610. doi: 10.1093/annonc/mdt575. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

