Quality of radiotherapy reporting in randomized controlled trials of prostate cancer
- PMID: 29880015
- PMCID: PMC5992848
- DOI: 10.1186/s13014-018-1053-7
Quality of radiotherapy reporting in randomized controlled trials of prostate cancer
Abstract
Background: Good radiotherapy reporting in clinical trials of prostate radiotherapy is important because it will allow accurate reproducibility of radiotherapy treatment and minimize treatment variations that can affect patient outcomes. The aim of our study is to assess the quality of prostate radiotherapy (RT) treatment reporting in randomized controlled trials in prostate cancer.
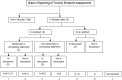
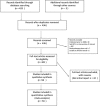
Methods: We searched MEDLINE for randomized trials of prostate cancer, published from 1996 to 2016 and included prostate RT as one of the intervention arms. We assessed if the investigators reported the ten criteria adequately in the trial reports: RT dose prescription method; RT dose-planning procedures; organs at risk (OAR) dose constraints; target volume definition, simulation procedures; treatment verification procedures; total RT dose; fractionation schedule; conduct of quality assurance (QA) as well as presence or absence of deviations in RT treatment planning and delivery. We performed multivariate logistic regression to determine the factors that may influence the quality of reporting.
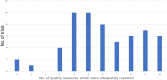
Results: We found 59 eligible trials. There was significant variability in the quality of reporting. Target volume definition, total RT dose and fractionation schedule were reported adequately in 97% of included trials. OAR constraints, simulation procedures and presence or absence of deviations in RT treatment planning and delivery were reported adequately in 30% of included trials. Twenty-four trials (40%) reported seven criteria or more adequately. Multivariable logistic analysis showed that trials that published their quality assurance results and cooperative group trials were more likely to have adequate quality in reporting in at least seven criteria.
Conclusion: There is significant variability in the quality of reporting on prostate radiotherapy treatment in randomized trials of prostate cancer. We need to have consensus guidelines to standardize the reporting of radiotherapy treatment in randomized trials.
Keywords: Prostate cancer; Quality of radiotherapy reporting; Randomized controlled trials.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Albert JM, Das P. Quality indicators in radiation oncology. Int J Radiation Oncol Biol Phys. 2013;85(4):904e911. - PubMed
-
- EQUATOR network. http://www.equator-network.org/reporting-guidelines/consort/. Accessed 20 Jul 2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

