Hexavalent Chromium-Induced Chromosome Instability Drives Permanent and Heritable Numerical and Structural Changes and a DNA Repair-Deficient Phenotype
- PMID: 29880483
- PMCID: PMC6072558
- DOI: 10.1158/0008-5472.CAN-18-0531
Hexavalent Chromium-Induced Chromosome Instability Drives Permanent and Heritable Numerical and Structural Changes and a DNA Repair-Deficient Phenotype
Abstract
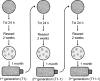
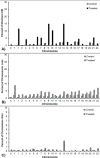
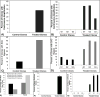
A key hypothesis for how hexavalent chromium [Cr(VI)] causes cancer is that it drives chromosome instability (CIN), which leads to neoplastic transformation. Studies show chronic Cr(VI) can affect DNA repair and induce centrosome amplification, which can lead to structural and numerical CIN. However, no studies have considered whether these outcomes are transient or permanent. In this study, we exposed human lung cells to particulate Cr(VI) for three sequential 24-hour periods, each separated by about a month. After each treatment, cells were seeded at colony-forming density, cloned, expanded, and retreated, creating three generations of clonal cell lines. Each generation of clones was tested for chromium sensitivity, chromosome complement, DNA repair capacity, centrosome amplification, and the ability to grow in soft agar. After the first treatment, Cr(VI)-treated clones exhibited a normal chromosome complement, but some clones showed a repair-deficient phenotype and amplified centrosomes. After the second exposure, more than half of the treated clones acquired an abnormal karyotype including numerical and structural alterations, with many exhibiting deficient DNA double-strand break repair and amplified centrosomes. The third treatment produced new abnormal clones, with previously abnormal clones acquiring additional abnormalities and most clones exhibiting repair deficiency. CIN, repair deficiency, and amplified centrosomes were all permanent and heritable phenotypes of repeated Cr(VI) exposure. These outcomes support the hypothesis that CIN is a key mechanism of Cr(VI)-induced carcinogenesis.Significance: Chromium, a major public health concern and human lung carcinogen, causes fundamental changes in chromosomes and DNA repair in human lung cells. Cancer Res; 78(15); 4203-14. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. “Hot spots” of chromium accumulation at bifurcations of chromate workers’ bronchi. Cancer Res. 1994a;54(9):2342–6. - PubMed
-
- Kondo K, Takahashi Y, Ishikawa S, Uchihara H, Hirose Y, Yoshizawa K, Tsuyuguchi M, Takizawa H, Miyoshi T, Sakiyama S, Monden Y. Microscopic analysis of chromium accumulation in the bronchi and lung of chromate workers. Cancer. 2003;98(11):2420–9. - PubMed
-
- Takahashi Y, Kondo K, Ishikawa S, Uchihara H, Fujino H, Sawada N, Miyoshi T, Sakiyama S, Izumi K, Monden Y. Microscopic analysis of the chromium content in the chromium-induced malignant and premalignant bronchial lesions of the rat. Environ Res. 2005a;99(2):267–72. - PubMed
-
- Hirose T, Kondo K, Takahashi Y, Ishikura H, Fujino H, Tsuyuguchi M, Hashimoto M, Yokose T, Mukai K, Kodama T, Monden Y. Frequent microsatellite instability in lung cancer from chromate-exposed workers. Mol Carcinog. 2002;33(3):172–80. - PubMed
-
- Takahashi Y, Kondo K, Hirose T, Nakagawa H, Tsuyuguchi M, Hashimoto M, Sano T, Ochiai A, Monden Y. Microsatellite instability and protein expression of the DNA mismatch repair gene, hMLH1, of lung cancer in chromate-exposed workers. Mol Carcinog. 2005b;42:150–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

