Comparative Effects of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Inhibition and Statins on Postprandial Triglyceride-Rich Lipoprotein Metabolism
- PMID: 29880491
- PMCID: PMC6039422
- DOI: 10.1161/ATVBAHA.118.310882
Comparative Effects of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Inhibition and Statins on Postprandial Triglyceride-Rich Lipoprotein Metabolism
Abstract
Objective: Inhibition of PCSK9 (proprotein convertase subtilisin/kexin type 9) and statins are known to lower plasma LDL (low-density lipoprotein)-cholesterol concentrations. However, the comparative effects of these treatments on the postprandial metabolism of TRLs (triglyceride-rich lipoproteins) remain to be investigated.
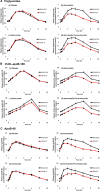
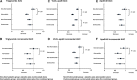
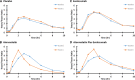
Approach and results: We performed a 2-by-2 factorial trial of the effects of 8 weeks of subcutaneous evolocumab (420 mg every 2 weeks) and atorvastatin (80 mg daily) on postprandial TRL metabolism in 80 healthy, normolipidemic men after ingestion of an oral fat load. We evaluated plasma total and incremental area under the curves for triglycerides, apo (apolipoprotein)B-48, and VLDL (very-LDL)-apoB-100. We also examined the kinetics of apoB-48 using intravenous D3-leucine administration, mass spectrometry, and multicompartmental modeling. Atorvastatin and evolocumab independently lowered postprandial VLDL-apoB-100 total area under the curves (P<0.001). Atorvastatin, but not evolocumab, reduced fasting plasma apoB-48, apoC-III, and angiopoietin-like 3 concentrations (P<0.01), as well as postprandial triglyceride and apoB-48 total area under the curves (P<0.001) and the incremental area under the curves for plasma triglycerides, apoB-48, and VLDL-apoB-100 (P<0.01). Atorvastatin also independently increased TRL apoB-48 fractional catabolic rate (P<0.001) and reduced the number of apoB-48-containing particles secreted in response to the fat load (P<0.01). In contrast, evolocumab did not significantly alter the kinetics of apoB-48.
Conclusions: In healthy, normolipidemic men, atorvastatin decreased fasting and postprandial apoB-48 concentration by accelerating the catabolism of apoB-48 particles and reducing apoB-48 particle secretion in response to a fat load. Inhibition of PCSK9 with evolocumab had no significant effect on apoB-48 metabolism.
Keywords: fasting; leucine; lipoproteins; mass spectrometry; triglycerides.
© 2018 American Heart Association, Inc.
Figures

References
-
- Havel RJ. Triglyceride-rich lipoproteins and plasma lipid transport. Arterioscler Thromb Vasc Biol. 2019;30:9–19. doi: 10.1161/ATVBAHA.108.178756. - PubMed
-
- Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. - PubMed
-
- Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:2525–2540. doi: 10.1016/j.jacc.2014.09.042. - PubMed
-
- Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. - PubMed
-
- Ginsberg HN, Le NA, Goldberg IJ, Gibson JC, Rubinstein A, Wang-Iverson P, Norum R, Brown WV. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest. 1986;78:1287–1295. doi: 10.1172/JCI112713. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

