Role of 3-Dimensional Architecture of Scar and Surviving Tissue in Ventricular Tachycardia: Insights From High-Resolution Ex Vivo Porcine Models
- PMID: 29880529
- PMCID: PMC5994925
- DOI: 10.1161/CIRCEP.117.006131
Role of 3-Dimensional Architecture of Scar and Surviving Tissue in Ventricular Tachycardia: Insights From High-Resolution Ex Vivo Porcine Models
Abstract
Background: An improved knowledge of the spatial organization of infarct structure and its contribution to ventricular tachycardia (VT) is important for designing optimal treatments. This study explores the relationship between the 3-dimensional structure of the healed infarct and the VT reentrant pathways in high-resolution models of infarcted porcine hearts.
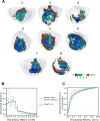
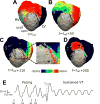
Methods: Structurally detailed models of infarcted ventricles were reconstructed from ex vivo late gadolinium enhancement and diffusion tensor magnetic resonance imaging data of 8 chronically infarcted porcine hearts at submillimeter resolution (0.25×0.25×0.5 mm3). To characterize the 3-dimensional structure of surviving tissue in the zone of infarct, a novel scar-mapped thickness metric was introduced. Further, using the ventricular models, electrophysiological simulations were conducted to determine and analyze the 3-dimensional VT pathways that were established in each of the complex infarct morphologies.
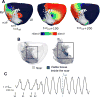
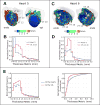
Results: The scar-mapped thickness metric revealed the heterogeneous organization of infarct and enabled us to systematically characterize the distribution of surviving tissue thickness in 8 hearts. Simulation results demonstrated the involvement of a subendocardial tissue layer of varying thickness in the majority of VT pathways. Importantly, they revealed that VT pathways are most frequently established within thin surviving tissue structures of thickness ≤2.2 mm (90th percentile) surrounding the scar.
Conclusions: The combination of high-resolution imaging data and ventricular simulations revealed the 3-dimensional distribution of surviving tissue surrounding the scar and demonstrated its involvement in VT pathways. The new knowledge obtained in this study contributes toward a better understanding of infarct-related VT.
Keywords: arrhythmias, cardiac; computer simulation; magnetic resonance imaging; myocardial infarction; tachycardia, ventricular.
© 2018 American Heart Association, Inc.
Figures



References
-
- John RM, Tedrow UB, Koplan BA, Albert CM, Epstein LM, Sweeney MO, Miller AL, Michaud GF, Stevenson WG. Ventricular arrhythmias and sudden cardiac death. Lancet. 2012;380:1520–1529. - PubMed
-
- Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. - PubMed
-
- Kléber AG, Rudy Y. Basic Mechanisms of Cardiac Impulse Propagation and Associated Arrhythmias. Physiol Rev. 2009;84:431–488. - PubMed
-
- Josephson ME. Clinical Cardiac Electrophysiology: Techniques and Interpretations. Fourth. Lippincott Williams & Wilkins; 2008. pp. 446–642.
-
- de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Slow conduction in the infarcted human heart. “Zigzag” course of activation. Circulation. 1993;88:915–926. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

