POP-Brazil study protocol: a nationwide cross-sectional evaluation of the prevalence and genotype distribution of human papillomavirus (HPV) in Brazil
- PMID: 29880568
- PMCID: PMC6009568
- DOI: 10.1136/bmjopen-2017-021170
POP-Brazil study protocol: a nationwide cross-sectional evaluation of the prevalence and genotype distribution of human papillomavirus (HPV) in Brazil
Abstract
Introduction: Human papillomavirus (HPV) is associated with the development of genital warts and different types of cancer, including virtually all cervical cancers and a considerable number of penile, anal and oropharyngeal cancers. Data regarding the prevalence of HPV infection in Brazil are limited and fragmented. We aim to determine HPV prevalence in sexually active women and men aged 16-25 years and to investigate regional differences in virus prevalence and types.
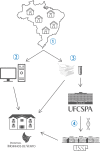
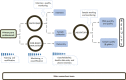
Methods and analysis: This is a nationwide, multicentric, cross-sectional, prospective study that will include participants aged 16-25 years from all Brazilian capital cities. Recruitment will occur in primary health units by trained health professionals who will be responsible for collecting biological samples and interviewing the volunteers. After signing informed consent, all participants will answer a questionnaire that will collect sociodemographic and behavioural data. All samples will be processed in a certified central laboratory, and strict quality control will be performed by many different procedures, including double data entry, training and certification of primary care health professionals responsible for data collection, simulation of interviews, and auditing and monitoring of visits. The sample size will be standardised based on the population distribution of each capital using SAS and R statistical software.
Ethics and dissemination: The project was approved by the research ethics committee of the main institution and the corresponding ethics committees of the recruitment sites. This will be the first Brazilian nationwide study to determine overall HPV prevalence and to examine regional differences and social, demographic and behavioural factors related to HPV infection. Critical analysis of the study results will contribute to epidemiological knowledge and will set a baseline for future evaluation of the impact of the National HPV Vaccination Program.
Keywords: Brazil; anogenital; hpv infection; nationwide study; prevalence.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: ASB, FMAdS, CD and AGKM work for the Ministry of Health of Brazil. LLV is a consultant for Merck for the HPV quadrivalent vaccine and for Qiagen, BD and Roche for HPV DNA tests.
Figures
References
-
- Globocan. Home. 2012. http://globocan.iarc.fr/Default.aspx (accessed 1 Dec 2017).
-
- Portal-Instituto Nacional de Câncer-INCA. INCA promove exposição sobre o câncer do colo do útero. http://www2.inca.gov.br/wps/wcm/connect/inca/portal/home (accessed 1 Dec 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
