Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2
- PMID: 29880603
- PMCID: PMC6119145
- DOI: 10.3324/haematol.2018.192328
Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2
Abstract
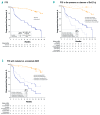
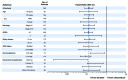
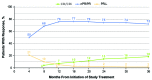
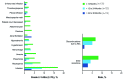
Results of RESONATE-2 (PCYC-1115/1116) supported approval of ibrutinib for first-line treatment of chronic lymphocytic leukemia. Extended analysis of RESONATE-2 was conducted to determine long-term efficacy and safety of ibrutinib in older patients with chronic lymphocytic leukemia. A total of 269 patients aged ≥65 years with previously untreated chronic lymphocytic leukemia without del(17p) were randomized 1:1 to ibrutinib (n=136) or chlorambucil (n=133) on days 1 and 15 of a 28-day cycle for 12 cycles. Median ibrutinib treatment duration was 28.5 months. Ibrutinib significantly prolonged progression-free survival versus chlorambucil (median, not reached vs 15 months; hazard ratio, 0.12; 95% confidence interval, 0.07-0.20; P<0.0001). The 24-month progression-free survival was 89% with ibrutinib (97% and 89% in patients with del[11q] and unmutated immunoglobulin heavy chain variable region gene, respectively). Progression-free survival rates at 24 months were also similar regardless of age (<75 years [88%], ≥75 years [89%]). Overall response rate was 92% (125/136). Rate of complete response increased substantially from 7% at 12 months to 18% with extended follow up. Greater quality of life improvements occurred with ibrutinib versus chlorambucil in Functional Assessment of Chronic Illness Therapy-Fatigue (P=0.0013). The most frequent grade ≥3 adverse events were neutropenia (12%), anemia (7%), and hypertension (5%). Rate of discontinuations due to adverse events was 12%. Results demonstrated that first-line ibrutinib for elderly patients with chronic lymphocytic leukemia provides sustained response and progression-free survival benefits over chemotherapy, with depth of response improving over time without new toxicity concerns. This trial was registered at clinicaltrials.gov identifier 01722487 and 01724346.
Trial registration: ClinicalTrials.gov NCT01722487 NCT01724346.
Copyright© 2018 Ferrata Storti Foundation.
Figures
References
-
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review 1975–2013. Bethesda, MD: National Cancer Institute; 2014. Available from: http://seer.cancer.gov/archive/csr/1975_2013/, based on November 2015 SEER data submission, posted to SEER web site, April 2016 Accessed March 17, 2017.
-
- Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–3391. - PubMed
-
- Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942. - PubMed
-
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. - PubMed
-
- de Gorter DJ, Beuling EA, Kersseboom R, et al. Bruton’s tyrosine kinase and phospholipase C 2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26(1):93–104. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

