Impaired mitochondrial activity explains platelet dysfunction in thrombocytopenic cancer patients undergoing chemotherapy
- PMID: 29880611
- PMCID: PMC6119160
- DOI: 10.3324/haematol.2017.185165
Impaired mitochondrial activity explains platelet dysfunction in thrombocytopenic cancer patients undergoing chemotherapy
Abstract
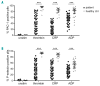
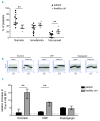
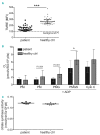
Severe thrombocytopenia (≤50×109 platelets/L) due to hematological malignancy and intensive chemotherapy is associated with an increased risk of clinically significant bleeding. Since the bleeding risk is not linked to the platelet count only, other hemostatic factors must be involved. We studied platelet function in 77 patients with acute leukemia, multiple myeloma or malignant lymphoma, who experienced chemotherapy-induced thrombocytopenia. Platelets from all patients - independent of disease or treatment type - were to a variable extent compromised in Ca2+ flux, integrin a β activation and P-selectin expression when stimulated with a panelIIbof3 agonists. The patients' platelets were also impaired in spreading on fibrinogen. Whereas the Ca2+ store content was unaffected, the patients' platelets showed ongoing phosphatidylserine exposure, which was not due to apoptotic caspase activity. Interestingly, mitochondrial function was markedly reduced in platelets from a representative subset of patients, as evidenced by a low mitochondrial membrane potential (P<0.001) and low oxygen consumption (P<0.05), while the mitochondrial content was normal. Moreover, the mitochondrial impairments coincided with elevated levels of reactive oxygen species (Spearman's rho=-0.459, P=0.012). Markedly, the impairment of platelet function only appeared after two days of chemotherapy, suggesting origination in the megakaryocytes. In patients with bone marrow recovery, platelet function improved. In conclusion, our findings disclose defective receptor signaling related to impaired mitochondrial bioenergetics, independent of apoptosis, in platelets from cancer patients treated with chemotherapy, explaining the low hemostatic potential of these patients.
Copyright© 2018 Ferrata Storti Foundation.
Figures



References
-
- Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327–358. - PubMed
-
- Apelseth TO, Hervig T, Bruserud Ø. Current practice and future directions for optimization of platelet transfusions in patients with severe therapy-induced cytopenia. Blood Rev. 2011;25(3):113–122. - PubMed
-
- Stanworth SJ, Estcourt LJ, Powter G, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368(19):1771–1780. - PubMed
-
- Wandt H, Schaefer-Eckart K, Wendeling K, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet. 2012;380(9850):1309–1316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

