Structure-based stabilization of insulin as a therapeutic protein assembly via enhanced aromatic-aromatic interactions
- PMID: 29880646
- PMCID: PMC6052209
- DOI: 10.1074/jbc.RA118.003650
Structure-based stabilization of insulin as a therapeutic protein assembly via enhanced aromatic-aromatic interactions
Abstract
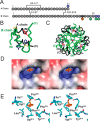
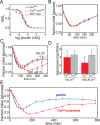
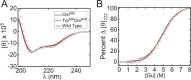
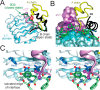
Key contributions to protein structure and stability are provided by weakly polar interactions, which arise from asymmetric electronic distributions within amino acids and peptide bonds. Of particular interest are aromatic side chains whose directional π-systems commonly stabilize protein interiors and interfaces. Here, we consider aromatic-aromatic interactions within a model protein assembly: the dimer interface of insulin. Semi-classical simulations of aromatic-aromatic interactions at this interface suggested that substitution of residue TyrB26 by Trp would preserve native structure while enhancing dimerization (and hence hexamer stability). The crystal structure of a [TrpB26]insulin analog (determined as a T3Rf3 zinc hexamer at a resolution of 2.25 Å) was observed to be essentially identical to that of WT insulin. Remarkably and yet in general accordance with theoretical expectations, spectroscopic studies demonstrated a 150-fold increase in the in vitro lifetime of the variant hexamer, a critical pharmacokinetic parameter influencing design of long-acting formulations. Functional studies in diabetic rats indeed revealed prolonged action following subcutaneous injection. The potency of the TrpB26-modified analog was equal to or greater than an unmodified control. Thus, exploiting a general quantum-chemical feature of protein structure and stability, our results exemplify a mechanism-based approach to the optimization of a therapeutic protein assembly.
Keywords: insulin; molecular dynamics; molecular pharmacology; protein design; protein self-assembly.
© 2018 Rege et al.
Conflict of interest statement
M. A. W. has equity in Thermalin, Inc. (Cleveland, OH) where he serves as Chief Innovation Officer; he has also been a consultant to Merck Research Laboratories and DEKA Research & Development Corp. N. F. B. P. is a consultant to Thermalin, Inc. F. I.-B. serves has equity in Thermalin, Inc. and is a consultant to Sanofi and Novo Nordisk
Figures





References
-
- Burley S. K., and Petsko G. A. (1988) Weakly polar interaction in proteins. Adv. Protein Chem. 39, 125–189 - PubMed
-
- Blundell T. L., Dodson G. G., Hodgkin D. C., and Mercola D. A. (1972) Insulin: the structure in the crystal and its reflection in chemistry and biology. Adv. Protein Chem. 26, 279–402 10.1016/S0065-3233(08)60143-6 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

