Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study
- PMID: 29880655
- PMCID: PMC6092680
- DOI: 10.1183/13993003.00766-2018
Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study
Abstract
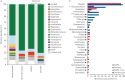
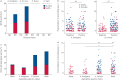
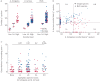
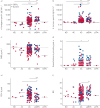
Understanding the composition and clinical importance of the fungal mycobiome was recently identified as a key topic in a "research priorities" consensus statement for bronchiectasis.Patients were recruited as part of the CAMEB study: an international multicentre cross-sectional Cohort of Asian and Matched European Bronchiectasis patients. The mycobiome was determined in 238 patients by targeted amplicon shotgun sequencing of the 18S-28S rRNA internally transcribed spacer regions ITS1 and ITS2. Specific quantitative PCR for detection of and conidial quantification for a range of airway Aspergillus species was performed. Sputum galactomannan, Aspergillus specific IgE, IgG and TARC (thymus and activation regulated chemokine) levels were measured systemically and associated to clinical outcomes.The bronchiectasis mycobiome is distinct and characterised by specific fungal genera, including Aspergillus, Cryptococcus and ClavisporaAspergillus fumigatus (in Singapore/Kuala Lumpur) and Aspergillus terreus (in Dundee) dominated profiles, the latter associating with exacerbations. High frequencies of Aspergillus-associated disease including sensitisation and allergic bronchopulmonary aspergillosis were detected. Each revealed distinct mycobiome profiles, and associated with more severe disease, poorer pulmonary function and increased exacerbations.The pulmonary mycobiome is of clinical relevance in bronchiectasis. Screening for Aspergillus-associated disease should be considered even in apparently stable patients.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: F.T. Chew reports personal fees from Sime Darby Technology Center, Olam International and First Resources Ltd, outside the submitted work. J.D. Chalmers reports grants from AstraZeneca, grants and personal fees from GlaxoSmithKline, Boehringer Ingelheim, Pfizer, Bayer Healthcare and Grifols, and personal fees from Napp, outside the submitted work.
Figures


References
-
- Polverino E, Goeminne PC, McDonnell MJ, et al. . European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. - PubMed
-
- Rogers GB, Zain NM, Bruce KD, et al. . A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc 2014; 11: 496–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
