Regulation of GnRH pulsatility in ewes
- PMID: 29880718
- PMCID: PMC6098716
- DOI: 10.1530/REP-18-0127
Regulation of GnRH pulsatility in ewes
Abstract
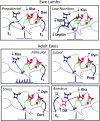
Early work in ewes provided a wealth of information on the physiological regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by internal and external inputs. Identification of the neural systems involved, however, was limited by the lack of information on neural mechanisms underlying generation of GnRH pulses. Over the last decade, considerable evidence supported the hypothesis that a group of neurons in the arcuate nucleus that contain kisspeptin, neurokinin B and dynorphin (KNDy neurons) are responsible for synchronizing secretion of GnRH during each pulse in ewes. In this review, we describe our current understanding of the neural systems mediating the actions of ovarian steroids and three external inputs on GnRH pulsatility in light of the hypothesis that KNDy neurons play a key role in GnRH pulse generation. In breeding season adults, estradiol (E2) and progesterone decrease GnRH pulse amplitude and frequency, respectively, by actions on KNDy neurons, with E2 decreasing kisspeptin and progesterone increasing dynorphin release onto GnRH neurons. In pre-pubertal lambs, E2 inhibits GnRH pulse frequency by decreasing kisspeptin and increasing dynorphin release, actions that wane as the lamb matures to allow increased pulsatile GnRH secretion at puberty. Less is known about mediators of undernutrition and stress, although some evidence implicates kisspeptin and dynorphin, respectively, in the inhibition of GnRH pulse frequency by these factors. During the anoestrus, inhibitory photoperiod acting via melatonin activates A15 dopaminergic neurons that innervate KNDy neurons; E2 increases dopamine release from these neurons to inhibit KNDy neurons and suppress the frequency of kisspeptin and GnRH release.
© 2018 Society for Reproduction and Fertility.
Conflict of interest statement
The authors have no conflict of interest that could be perceived as prejudicing the impartiality of the research present in this review.
Figures
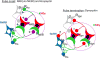



References
-
- Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22:1–12. - PMC - PubMed
-
- Anderson GM, Connors JM, Hardy SL, Valent M, Goodman RL. Oestradiol microimplants in the ventromedial preoptic area inhibit secretion of luteinizing hormone via dopamine neurones in anoestrous ewes. J Neuroendocrinol. 2001;13:1051–1058. - PubMed
-
- Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology. 2009;150:5488–5497. - PubMed
-
- Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010b;151:2233–2243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

