Loss of Drosha underlies dopaminergic neuron toxicity in models of Parkinson's disease
- PMID: 29880811
- PMCID: PMC5992196
- DOI: 10.1038/s41419-018-0716-5
Loss of Drosha underlies dopaminergic neuron toxicity in models of Parkinson's disease
Abstract
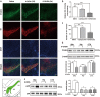
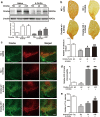
MiRNAs, a group of powerful modulator of gene expression, participate in multiple cellular processes under physiological and pathological conditions. Emerging evidence shows that Drosha, which controls the initial step in canonical miRNA biogenesis, is involved in modulating cell survival and death in models of several diseases. However, the role of Drosha in Parkinson's disease (PD) has not been well established. Here, we show that the level of Drosha decreases in 6-OHDA-induced cellular and animal models of PD. 6-OHDA induced a p38 MAPK-dependent phosphorylation of Drosha. This triggered Drosha degradation. Enhancing the level of Drosha protected the dopaminergic (DA) neurons from 6-OHDA-induced toxicity in both in vitro and in vivo models of PD and alleviated the motor deficits of PD mice. These findings reveal that Drosha plays a critical role in the survival of DA neurons and suggest that stress-induced destabilization of Drosha may be part of the pathological process in PD.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Stress induces p38 MAPK-mediated phosphorylation and inhibition of Drosha-dependent cell survival.Mol Cell. 2015 Feb 19;57(4):721-734. doi: 10.1016/j.molcel.2015.01.004. Mol Cell. 2015. PMID: 25699712 Free PMC article.
-
p38 MAPK-mediated loss of nuclear RNase III enzyme Drosha underlies amyloid beta-induced neuronal stress in Alzheimer's disease.Aging Cell. 2021 Oct;20(10):e13434. doi: 10.1111/acel.13434. Epub 2021 Sep 16. Aging Cell. 2021. PMID: 34528746 Free PMC article.
-
The Effect of Human Umbilical Cord Mesenchymal Stromal Cells in Protection of Dopaminergic Neurons from Apoptosis by Reducing Oxidative Stress in the Early Stage of a 6-OHDA-Induced Parkinson's Disease Model.Cell Transplant. 2019 Dec;28(1_suppl):87S-99S. doi: 10.1177/0963689719891134. Epub 2019 Nov 28. Cell Transplant. 2019. PMID: 31775521 Free PMC article.
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
-
Synaptic dysfunction in Parkinson's disease.Adv Exp Med Biol. 2012;970:553-72. doi: 10.1007/978-3-7091-0932-8_24. Adv Exp Med Biol. 2012. PMID: 22351072 Review.
Cited by
-
MiR-421 Aggravates Neurotoxicity and Promotes Cell Death in Parkinson's Disease Models by Directly Targeting MEF2D.Neurochem Res. 2021 Feb;46(2):299-308. doi: 10.1007/s11064-020-03166-0. Epub 2020 Nov 12. Neurochem Res. 2021. PMID: 33179210
-
The Impact of Dysregulated microRNA Biogenesis Machinery and microRNA Sorting on Neurodegenerative Diseases.Int J Mol Sci. 2023 Feb 8;24(4):3443. doi: 10.3390/ijms24043443. Int J Mol Sci. 2023. PMID: 36834853 Free PMC article. Review.
-
The 3' UTR polymorphisms rs3742330 in DICER1 and rs10719 in DROSHA genes are not associated with primary open-angle and angle-closure glaucoma: As case-control study.PLoS One. 2023 Apr 26;18(4):e0284852. doi: 10.1371/journal.pone.0284852. eCollection 2023. PLoS One. 2023. PMID: 37099569 Free PMC article.
-
An Emerging Role of miRNAs in Neurodegenerative Diseases: Mechanisms and Perspectives on miR146a.Antioxid Redox Signal. 2021 Sep 1;35(7):580-594. doi: 10.1089/ars.2020.8256. Epub 2021 Feb 15. Antioxid Redox Signal. 2021. PMID: 33403895 Free PMC article. Review.
-
Comparative Proteomics Highlights that GenX Exposure Leads to Metabolic Defects and Inflammation in Astrocytes.Environ Sci Technol. 2024 Nov 19;58(46):20525-20539. doi: 10.1021/acs.est.4c05472. Epub 2024 Nov 5. Environ Sci Technol. 2024. PMID: 39499804 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

