Parasitic insect-derived miRNAs modulate host development
- PMID: 29880839
- PMCID: PMC5992160
- DOI: 10.1038/s41467-018-04504-1
Parasitic insect-derived miRNAs modulate host development
Abstract
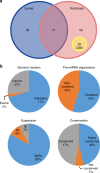
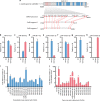
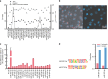
Parasitic wasps produce several factors including venom, polydnaviruses (PDVs) and specialized wasp cells named teratocytes that benefit the survival of offspring by altering the physiology of hosts. However, the underlying molecular mechanisms for the alterations remain unclear. Here we find that the teratocytes of Cotesia vestalis, an endoparasitoid of the diamondback moth Plutella xylostella, and its associated bracovirus (CvBV) can produce miRNAs and deliver the products into the host via different ways. Certain miRNAs in the parasitized host are mainly produced by teratocytes, while the expression level of miRNAs encoded by CvBV can be 100-fold greater in parasitized hosts than non-parasitized ones. We further show that one teratocyte-produced miRNA (Cve-miR-281-3p) and one CvBV-produced miRNA (Cve-miR-novel22-5p-1) arrest host growth by modulating expression of the host ecdysone receptor (EcR). Altogether, our results show the first evidence of cross-species regulation by miRNAs in animal parasitism and their possible function in the alteration of host physiology during parasitism.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Parasitic interference.Nat Rev Mol Cell Biol. 2018 Aug;19(8):487. doi: 10.1038/s41580-018-0035-9. Nat Rev Mol Cell Biol. 2018. PMID: 29942031 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

