Inflammation, neurodegeneration and protein aggregation in the retina as ocular biomarkers for Alzheimer's disease in the 3xTg-AD mouse model
- PMID: 29880901
- PMCID: PMC5992214
- DOI: 10.1038/s41419-018-0740-5
Inflammation, neurodegeneration and protein aggregation in the retina as ocular biomarkers for Alzheimer's disease in the 3xTg-AD mouse model
Abstract
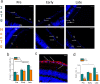
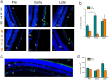
Alzheimer's disease (AD) is the most common cause of dementia in the elderly. In the pathogenesis of AD a pivotal role is played by two neurotoxic proteins that aggregate and accumulate in the central nervous system: amyloid beta and hyper-phosphorylated tau. Accumulation of extracellular amyloid beta plaques and intracellular hyper-phosphorylated tau tangles, and consequent neuronal loss begins 10-15 years before any cognitive impairment. In addition to cognitive and behavioral deficits, sensorial abnormalities have been described in AD patients and in some AD transgenic mouse models. Retina can be considered a simple model of the brain, as some pathological changes and therapeutic strategies from the brain may be observed or applicable to the retina. Here we propose new retinal biomarkers that could anticipate the AD diagnosis and help the beginning and the follow-up of possible future treatments. We analyzed retinal tissue of triple-transgenic AD mouse model (3xTg-AD) for the presence of pathological hallmarks during disease progression. We found the presence of amyloid beta plaques, tau tangles, neurodegeneration, and astrogliosis in the retinal ganglion cell layer of 3xTg-AD mice, already at pre-symptomatic stage. Moreover, retinal microglia in pre-symptomatic mice showed a ramified, anti-inflammatory phenotype which, during disease progression, switches to a pro-inflammatory, less ramified one, becoming neurotoxic. We hypothesize retina as a window through which monitor AD-related neurodegeneration process.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- WHO. Dementia: a Public Health Priority (World Health Organization, Geneva, 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

