Infection of Fungi and Bacteria in Brain Tissue From Elderly Persons and Patients With Alzheimer's Disease
- PMID: 29881346
- PMCID: PMC5976758
- DOI: 10.3389/fnagi.2018.00159
Infection of Fungi and Bacteria in Brain Tissue From Elderly Persons and Patients With Alzheimer's Disease
Abstract
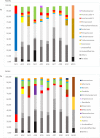
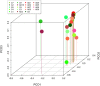
Alzheimer's disease (AD) is the leading cause of dementia in elderly people. The etiology of this disease remains a matter of intensive research in many laboratories. We have advanced the idea that disseminated fungal infection contributes to the etiology of AD. Thus, we have demonstrated that fungal proteins and DNA are present in nervous tissue from AD patients. More recently, we have reported that bacterial infections can accompany these mycoses, suggesting that polymicrobial infections exist in AD brains. In the present study, we have examined fungal and bacterial infection in brain tissue from AD patients and control subjects by immunohistochemistry. In addition, we have documented the fungal and bacterial species in brain regions from AD patients and control subjects by next-generation sequencing (NGS). Our results from the analysis of ten AD patients reveal a variety of fungal and bacterial species, although some were more prominent than others. The fungal genera more prevalent in AD patients were Alternaria, Botrytis, Candida, and Malassezia. We also compared these genera with those found in elderly and younger subjects. One of the most prominent genera in control subjects was Fusarium. Principal component analysis clearly indicated that fungi from frontal cortex samples of AD brains clustered together and differed from those of equivalent control subjects. Regarding bacterial infection, the phylum Proteobacteria was the most prominent in both AD patients and controls, followed by Firmicutes, Actinobacteria, and Bacteroides. At the family level, Burkholderiaceae and Staphylococcaceae exhibited higher percentages in AD brains than in control brains. These findings could be of interest to guide targeted antimicrobial therapy for AD patients. Moreover, the variety of microbial species in each patient may constitute a basis for a better understanding of the evolution and severity of clinical symptoms in each patient.
Keywords: Alzheimer’s disease; bacteria and fungal co-infections; fungal infection; infection in aging brain; next generation sequencing; polymicrobial infection.
Figures










References
LinkOut - more resources
Full Text Sources
Other Literature Sources

