Inhibition of the Activin Receptor Type-2B Pathway Restores Regenerative Capacity in Satellite Cell-Depleted Skeletal Muscle
- PMID: 29881353
- PMCID: PMC5978452
- DOI: 10.3389/fphys.2018.00515
Inhibition of the Activin Receptor Type-2B Pathway Restores Regenerative Capacity in Satellite Cell-Depleted Skeletal Muscle
Abstract
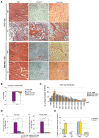
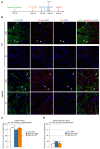
Degenerative myopathies typically display a decline in satellite cells coupled with a replacement of muscle fibers by fat and fibrosis. During this pathological remodeling, satellite cells are present at lower numbers and do not display a proper regenerative function. Whether a decline in satellite cells directly contributes to disease progression or is a secondary result is unknown. In order to dissect these processes, we used a genetic model to reduce the satellite cell population by ~70-80% which leads to a nearly complete loss of regenerative potential. We observe that while no overt tissue damage is observed following satellite cell depletion, muscle fibers atrophy accompanied by changes in the stem cell niche cellular composition. Treatment of these mice with an Activin receptor type-2B (AcvR2B) pathway blocker reverses muscle fiber atrophy as expected, but also restores regenerative potential of the remaining satellite cells. These findings demonstrate that in addition to controlling fiber size, the AcvR2B pathway acts to regulate the muscle stem cell niche providing a more favorable environment for muscle regeneration.
Keywords: TGFβ signaling; muscle atrophy; muscle regeneration; muscle stem cell niche; satellite cells.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

