Food-Anticipatory Behavior in Neonatal Rabbits and Rodents: An Update on the Role of Clock Genes
- PMID: 29881373
- PMCID: PMC5976783
- DOI: 10.3389/fendo.2018.00266
Food-Anticipatory Behavior in Neonatal Rabbits and Rodents: An Update on the Role of Clock Genes
Abstract
In mammals, the suprachiasmatic nucleus (SCN), the master circadian clock, is mainly synchronized to the environmental light/dark cycle. SCN oscillations are maintained by a molecular clockwork in which certain genes, Period 1-2, Cry1-2, Bmal1, and Clock, are rhythmically expressed. Disruption of these genes leads to a malfunctioning clockwork and behavioral and physiological rhythms are altered. In addition to synchronization of circadian rhythms by light, when subjects are exposed to food for a few hours daily, behavioral and physiological rhythms are entrained to anticipate mealtime, even in the absence of the SCN. The presence of anticipatory rhythms synchronized by food suggests the existence of an SCN-independent circadian pacemaker that might be dependent on clock genes. Interestingly, rabbit pups, unable to perceive light, suckle milk once a day, which entrains behavioral rhythms to anticipate nursing time. Mutations of clock genes, singly or in combination, affect diverse rhythms in brain activity and physiological processes, but anticipatory behavior and physiology to feeding time remains attenuated or unaffected. It had been suggested that compensatory upregulation of paralogs or subtypes genes, or even non-transcriptional mechanisms, are able to maintain circadian oscillations entrained to mealtime. In the present mini-review, we evaluate the current state of the role played by clock genes in meal anticipation and provide evidence for rabbit pups as a natural model of food-anticipatory circadian behavior.
Keywords: PER1 protein; circadian rhythms; clock gene mutant; corticosterone; food entrainment; restricted feeding.
Figures
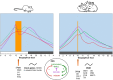
 ), corticosterone (
), corticosterone ( ), and body temperature (
), and body temperature ( ) increase in anticipation of the time of feeding in both species. The molecular clock is comprised of two principal feedback loops for the expression of clock genes. In the positive loop (green) the proteins CLOCK/NPAS2 and BMAL1 act on the transcription sites of Per, Cry, and Rev-erbα genes to induce their mRNA expression. Once the final proteins of PER and CRY (negative loop; red) are produced, these have the ability to repress their own transcription via an inhibitory action on the Clock-Npas2/Bmal1 dimer. The REV-ERBα protein is a transcriptional repressor for the Bmal1 gene driving rhythmic Bmal1. FOS protein expression and rhythms of clock genes and proteins in several brain nuclei synchronize to mealtime. DMH, dorsomedial hypothalamic nucleus; LH, lateral hypothalamus; MnPO, median preoptic nucleus; OB, olfactory bulb; OVLT, organum vasculosum of lamina terminalis; PeF, perifornical nucleus; paraventricular nucleus; SON, supraoptic nucleus; TM, tuberomammillary nucleus. Vertical bar and big arrow, feeding time. Figure derived from data previously published by: Angeles-Castellanos et al. (43), Caba et al. (37), Escobar et al. (44), Honma et al. (42), Jilge et al. (45), Mistlberger (7), Morgado et al. (40, 41, 46), and Rovirosa et al. (39).
) increase in anticipation of the time of feeding in both species. The molecular clock is comprised of two principal feedback loops for the expression of clock genes. In the positive loop (green) the proteins CLOCK/NPAS2 and BMAL1 act on the transcription sites of Per, Cry, and Rev-erbα genes to induce their mRNA expression. Once the final proteins of PER and CRY (negative loop; red) are produced, these have the ability to repress their own transcription via an inhibitory action on the Clock-Npas2/Bmal1 dimer. The REV-ERBα protein is a transcriptional repressor for the Bmal1 gene driving rhythmic Bmal1. FOS protein expression and rhythms of clock genes and proteins in several brain nuclei synchronize to mealtime. DMH, dorsomedial hypothalamic nucleus; LH, lateral hypothalamus; MnPO, median preoptic nucleus; OB, olfactory bulb; OVLT, organum vasculosum of lamina terminalis; PeF, perifornical nucleus; paraventricular nucleus; SON, supraoptic nucleus; TM, tuberomammillary nucleus. Vertical bar and big arrow, feeding time. Figure derived from data previously published by: Angeles-Castellanos et al. (43), Caba et al. (37), Escobar et al. (44), Honma et al. (42), Jilge et al. (45), Mistlberger (7), Morgado et al. (40, 41, 46), and Rovirosa et al. (39).References
-
- Moore RY. The suprachiasmatic nucleus and the circadian timing system. In: Guillette MU. editor. Progress in Molecular Biology and Translational Science. (Vol. 119), Elsevier; (2013). p. 1–28. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

