Whole genome sequence revealed the fine transmission map of carbapenem-resistant Klebsiella pneumonia isolates within a nosocomial outbreak
- PMID: 29881543
- PMCID: PMC5984795
- DOI: 10.1186/s13756-018-0363-8
Whole genome sequence revealed the fine transmission map of carbapenem-resistant Klebsiella pneumonia isolates within a nosocomial outbreak
Abstract
Background: Carbapenem-resistant Klebsiella pneumoniae (CRKP) is a major cause of nosocomial infections worldwide. The transmission route of CRKP isolates within an outbreak is rarely described. This study aimed to reveal the molecular characteristics and transmission route of CRKP isolates within an outbreak of nosocomial infection.
Methods: Collecting case information, active screening and targeted environmental monitoring were carried out. The antibiotic susceptibility, drug-resistant genes, molecular subtype and whole genome sequence of CRKP strains were analyzed.
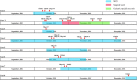
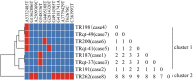
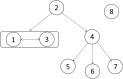
Results: Between October and December 2011, 26 CRKP isolates were collected from eight patients in a surgical intensive care unit and subsequent transfer wards of Beijing Tongren hospital, China. All 26 isolates harbored blaKPC-2, blaSHV-1, and blaCTX-M-15 genes, had the same or similar pulsed-field gel electrophoresis patterns, and belonged to the sequence type 11 (ST11) clone. By comprehensive consideration of genomic and epidemiological information, a putative transmission map was constructed, including identifying one case as an independent event distinct from the other seven cases, and revealing two transmissions starting from the same case.
Conclusions: This study provided the first report confirming an outbreak caused by K. pneumoniae ST11 clone co-harboring the blaKPC-2, blaCTX-M-15, and blaSHV-1 genes, and suggested that comprehensive consideration of genomic and epidemiological data can yield a fine transmission map of an outbreak and facilitate the control of nosocomial transmission.
Keywords: Carbapenemases; K. pneumoniae; KPC-2; Outbreak; Whole genome sequencing.
Conflict of interest statement
This study was approved by the scientific and ethics committees of Beijing Tongren Hospital. All clinical specimens from patients were collected for diagnostic testing in hospitals at the request of the attending doctors. The active screening and targeted environmental monitoring was carried out in the case of an emergency investigation and in accordance with the recommendations of “Emergency treatment plan for hospital infection outbreak in Tongren Hospital” and “2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (https://www.cdc.gov/infectioncontrol/pdf/guidelines/isolation-guidelines.pdf)”. All experiments were performed in accordance with relevant guidelines and regulations. The consent of the patients for the active screening of specimens, including K. pneumoniae detection, was obtained verbally by medical staff in the hospital. The medical records were considered as legal documents. Furthermore, to protect patient privacy, the hospital set up a patient privacy and medical record management system according to the criminal procedure law, civil procedure law, tort liability law, and medical malpractice law in China. Excepting for the patient’s doctor, no one can enter the management system. Although no written informed consent was provided by patients, the above measures are sufficient to protect the patients’ privacy.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Grundmann H, Livermore DM, Giske CG, Canton R, Rossolini GM, Campos J, et al. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill. 2010;15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

