Microenvironment-Driven Dynamic Heterogeneity and Phenotypic Plasticity as a Mechanism of Melanoma Therapy Resistance
- PMID: 29881716
- PMCID: PMC5976798
- DOI: 10.3389/fonc.2018.00173
Microenvironment-Driven Dynamic Heterogeneity and Phenotypic Plasticity as a Mechanism of Melanoma Therapy Resistance
Abstract
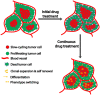
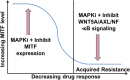
Drug resistance constitutes a major challenge in designing melanoma therapies. Microenvironment-driven tumor heterogeneity and plasticity play a key role in this phenomenon. Melanoma is highly heterogeneous with diverse genomic alterations and expression of different biological markers. In addition, melanoma cells are highly plastic and capable of adapting quickly to changing microenvironmental conditions. These contribute to variations in therapy response and durability between individual melanoma patients. In response to changing microenvironmental conditions, like hypoxia and nutrient starvation, proliferative melanoma cells can switch to an invasive slow-cycling state. Cells in this state are more aggressive and metastatic, and show increased intrinsic drug resistance. During continuous treatment, slow-cycling cells are enriched within the tumor and give rise to a new proliferative subpopulation with increased drug resistance, by exerting their stem cell-like behavior and phenotypic plasticity. In melanoma, the proliferative and invasive states are defined by high and low microphthalmia-associated transcription factor (MITF) expression, respectively. It has been observed that in MITFhigh melanomas, inhibition of MITF increases the efficacy of targeted therapies and delays the acquisition of drug resistance. Contrarily, MITF is downregulated in melanomas with acquired drug resistance. According to the phenotype switching theory, the gene expression profile of the MITFlow state is predominantly regulated by WNT5A, AXL, and NF-κB signaling. Thus, different combinations of therapies should be effective in treating different phases of melanoma, such as the combination of targeted therapies with inhibitors of MITF expression during the initial treatment phase, but with inhibitors of WNT5A/AXL/NF-κB signaling during relapse.
Keywords: cancer drug resistance; clonality; melanoma; microphthalmia-associated transcription factor; slow-cycling tumor cells; tumor heterogeneity; tumor microenvironment; tumor plasticity.
Figures
References
-
- Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov (2014) 4:80–93.10.1158/2159-8290.CD-13-0642 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

