Physico-Chemical Properties of MgGa Mixed Oxides and Reconstructed Layered Double Hydroxides and Their Performance in Aldol Condensation of Furfural and Acetone
- PMID: 29881721
- PMCID: PMC5976797
- DOI: 10.3389/fchem.2018.00176
Physico-Chemical Properties of MgGa Mixed Oxides and Reconstructed Layered Double Hydroxides and Their Performance in Aldol Condensation of Furfural and Acetone
Abstract
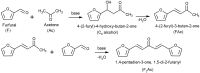
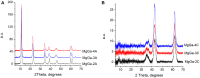
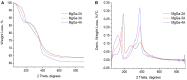
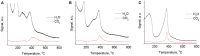
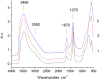
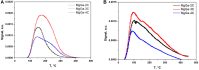
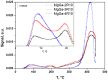
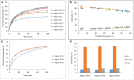
MgGa layered double hydroxides (Mg/Ga = 2-4) were synthesized and used for the preparation of MgGa mixed oxides and reconstructed hydrotalcites. The properties of the prepared materials were examined by physico-chemical methods (XRD, TGA, NH3-TPD, CO2-TPD, SEM, and DRIFT) and tested in aldol condensation of furfural and acetone. The as-prepared phase-pure MgGa samples possessed hydrotalcite structure, and their calcination resulted in mixed oxides with MgO structure with a small admixture phase characterized by a reflection at 2θ ≈ 36.0°. The interaction of MgGa mixed oxides with pure water resulted in reconstruction of the HTC structure already after 15 s of the rehydration with maximum crystallinity achieved after 60 s. TGA-MS experiments proved a substantial decrease in carbonates in all rehydrated samples compared with their as-prepared counterparts. This allowed suggesting presence of interlayer hydroxyls in the samples. Acido-basic properties of MgGa mixed oxides determined by TPD technique did not correlate with Mg/Ga ratio which was explained by the specific distribution of Ga atoms on the external surface of the samples. CO2-TPD method was also used to evaluate the basic properties of the reconstructed MgGa samples. In these experiments, an intensive peak at T = 450°C on CO2-TPD curve was attributed to the decomposition of carbonates newly formed by CO2 interaction with interlayer carbonates rather than to CO2 desorption from basic sites. Accordingly, CO2-TPD method quantitatively characterized the interlayer hydroxyls only indirectly. Furfural conversion on reconstructed MgGa materials was much larger compared with MgGa mixed oxides confirming that Brønsted basic sites in MgGa catalysts, like MgAl catalysts, were active in the reaction. Mg/Ga ratio in mixed oxides influenced product selectivity which was explained by the difference in textural properties of the samples. In contrast, Mg/Ga ratio in reconstructed catalysts had practically no effect on the composition of reaction products suggesting that the basic sites in these catalysts acted similarly in aldol condensation of acetone with furfural. It was concluded that the properties of MgGa samples resembled in a great extent those of MgAl hydrotalcite-based materials and demonstrated their potential as catalysts for base-catalyzed reactions.
Keywords: MgGa layered double hydroxides; acido-basic properties; aldol condensation; mixed oxides; reconstructed hydrotalcites.
Figures




References
-
- Allegra G., Ronca G. (1978). Crystal powder statistics. II. Line profiles in diffraction spectra of identical crystals and of Gaussian samples. Crystal size distributions. Acta Crystallogr. Sect. A 34, 1006–1013. 10.1107/S0567739478002053 - DOI
-
- Aramenda M. A., Borau V., Jimenez C., Marinas J. M., Ruiz J. R., Urbano F. J. (2003). Catalytic hydrogen transfer from 2-propanol to cyclohexanone over basic Mg–Al oxides. Appl. Catal. A Gen. 255, 301–308. 10.1016/S0926-860X(03)00569-6 - DOI
-
- Aramendía M. A., Avilés Y., Benítez J. A., Borau V., Jiménez C., Marinas J. M., et al. (1999a). Comparative study of Mg/Al and Mg/Ga layered double hydroxides. Micropor. Mesopor. Mater. 29, 319–328. 10.1016/S1387-1811(98)00345-X - DOI
-
- Aramendía M. A., Avilés Y., Borau V., Luque J. M., Marinas J. M., Ruiz J. R., et al. (1999b). Thermal decomposition of Mg/Al and Mg/Ga layered-double hydroxides: a spectroscopic study. J. Mater. Chem. 9, 1603–1607. 10.1039/a900535h - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

