Increased susceptibility to cardiovascular disease in offspring born from dams of advanced maternal age
- PMID: 29882308
- PMCID: PMC6265548
- DOI: 10.1113/JP275472
Increased susceptibility to cardiovascular disease in offspring born from dams of advanced maternal age
Abstract
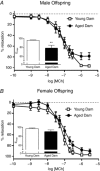
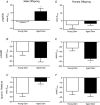
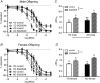
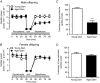
Key points: Advanced maternal age increases the risk of pregnancy complications such as fetal growth restriction, hypertension and premature birth. Offspring born from compromised pregnancies are at increased risk of cardiovascular disease as adults. However, the effect of advanced maternal age on later-onset disease in offspring has not been investigated. In adulthood, male but not female offspring born to dams of advanced maternal age showed impaired recovery from cardiac ischaemia/reperfusion injury. Endothelium-dependent relaxation was also impaired in male but not female offspring born from aged dams. Oxidative stress may play a role in the developmental programming of cardiovascular disease in this model. Given the increasing trend toward delayed parenthood, these findings have significant population and health care implications and warrant further investigation.
Abstract: Exposure to prenatal stressors, including hypoxia, micro- and macronutrient deficiency, and maternal stress, increases the risk of cardiovascular disease in adulthood. It is unclear whether being born from a mother of advanced maternal age (≥35 years old) may also constitute a prenatal stress with cardiovascular consequences in adulthood. We previously demonstrated growth restriction in fetuses from a rat model of advanced maternal age, suggesting exposure to a compromised in utero environment. Thus, we hypothesized that male and female offspring from aged dams would exhibit impaired cardiovascular function as adults. In 4-month-old offspring, we observed impaired endothelium-dependent relaxation in male (P < 0.05) but not female offspring born from aged dams. The anti-oxidant polyethylene glycol superoxide dismutase improved relaxation only in arteries from male offspring of aged dams (ΔEmax : young dam -1.63 ± 0.80 vs. aged dam 11.75 ± 4.23, P < 0.05). Furthermore, endothelium-derived hyperpolarization-dependent relaxation was reduced in male but not female offspring of aged dams (P < 0.05). Interestingly, there was a significant increase in nitric oxide contribution to relaxation in females born from aged dams (ΔEmax : young dam -24.8 ± 12.1 vs. aged dam -68.7 ± 7.7, P < 0.05), which was not observed in males. Recovery of cardiac function following an ischaemia-reperfusion insult in male offspring born from aged dams was reduced by ∼57% (P < 0.001), an effect that was not evident in female offspring. These data indicate that offspring born from aged dams have an altered cardiovascular risk profile that is sex-specific. Given the increasing trend toward delaying pregnancy, these findings may have significant population and health care implications and warrant further investigation.
Keywords: advanced maternal age; cardiovascular dysfunction; developmental programming.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures


Similar articles
-
Sex-specific effects of advanced maternal age on cardiovascular function in aged adult rat offspring.Am J Physiol Heart Circ Physiol. 2018 Dec 1;315(6):H1724-H1734. doi: 10.1152/ajpheart.00375.2018. Epub 2018 Oct 5. Am J Physiol Heart Circ Physiol. 2018. PMID: 30289293
-
Hyperglycaemia in pregnant rats causes sex-related vascular dysfunction in adult offspring: role of cyclooxygenase-2.Exp Physiol. 2017 Aug 1;102(8):1019-1036. doi: 10.1113/EP086132. Epub 2017 Jul 5. Exp Physiol. 2017. PMID: 28556994
-
The effect of tauroursodeoxycholic Acid (TUDCA) treatment on placental endoplasmic reticulum (ER) stress in a rat model of advanced maternal age.PLoS One. 2023 Apr 6;18(4):e0282442. doi: 10.1371/journal.pone.0282442. eCollection 2023. PLoS One. 2023. PMID: 37023067 Free PMC article.
-
Advanced maternal age and the impact on maternal and offspring cardiovascular health.Am J Physiol Heart Circ Physiol. 2019 Aug 1;317(2):H387-H394. doi: 10.1152/ajpheart.00045.2019. Epub 2019 Jun 14. Am J Physiol Heart Circ Physiol. 2019. PMID: 31199185 Review.
-
Programming of maternal and offspring disease: impact of growth restriction, fetal sex and transmission across generations.J Physiol. 2016 Sep 1;594(17):4727-40. doi: 10.1113/JP271745. Epub 2016 Apr 24. J Physiol. 2016. PMID: 26970222 Free PMC article. Review.
Cited by
-
Early Life Oxidative Stress and Long-Lasting Cardiovascular Effects on Offspring Conceived by Assisted Reproductive Technologies: A Review.Int J Mol Sci. 2020 Jul 22;21(15):5175. doi: 10.3390/ijms21155175. Int J Mol Sci. 2020. PMID: 32707756 Free PMC article. Review.
-
The Influence of Maternal Condition on Fetal Cardiac Function during the Second Trimester.Diagnostics (Basel). 2023 Aug 25;13(17):2755. doi: 10.3390/diagnostics13172755. Diagnostics (Basel). 2023. PMID: 37685293 Free PMC article.
-
Paternal metabolic and cardiovascular programming of their offspring: A systematic scoping review.PLoS One. 2020 Dec 31;15(12):e0244826. doi: 10.1371/journal.pone.0244826. eCollection 2020. PLoS One. 2020. PMID: 33382823 Free PMC article.
-
Impact of Stress and Anxiety on Cardiovascular Health in Pregnancy: A Scoping Review.J Clin Med. 2025 Jan 30;14(3):909. doi: 10.3390/jcm14030909. J Clin Med. 2025. PMID: 39941580 Free PMC article. Review.
-
[Mechanisms of the Effect of Maternal Age-Related Oocyte Aging on Fertility: Transcriptomic Sequencing Analysis of a Zebrafish Model].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 May 20;55(3):588-595. doi: 10.12182/20240560205. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38948296 Free PMC article. Chinese.
References
-
- Allison BJ, Kaandorp JJ, Kane AD, Camm EJ, Lusby C, Cross CM, Nevin‐Dolan R, Thakor AS, Derks JB, Tarry‐Adkins JL, Ozanne SE & Giussani DA (2016). Divergence of mechanistic pathways mediating cardiovascular aging and developmental programming of cardiovascular disease. FASEB J 30, 1968–1975. - PMC - PubMed
-
- Barker D (1994). Mother, Babies and Disease in Later Life. BMJ Publishing Group, London.
-
- Barker DJ (1992). The fetal origins of adult hypertension. J Hypertens Suppl 10, S39–S44. - PubMed
-
- Barker DJ (2000). In utero programming of cardiovascular disease. Theriogenology 53, 555–574. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

