Stomatal Complex Development and F-Actin Organization in Maize Leaf Epidermis Depend on Cellulose Synthesis
- PMID: 29882773
- PMCID: PMC6099634
- DOI: 10.3390/molecules23061365
Stomatal Complex Development and F-Actin Organization in Maize Leaf Epidermis Depend on Cellulose Synthesis
Abstract
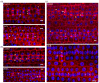
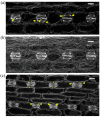
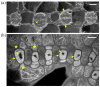
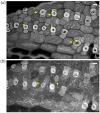
Cellulose microfibrils reinforce the cell wall for morphogenesis in plants. Herein, we provide evidence on a series of defects regarding stomatal complex development and F-actin organization in Zea mays leaf epidermis, due to inhibition of cellulose synthesis. Formative cell divisions of stomatal complex ontogenesis were delayed or inhibited, resulting in lack of subsidiary cells and frequently in unicellular stomata, with an atypical stomatal pore. Guard cells failed to acquire a dumbbell shape, becoming rounded, while subsidiary cells, whenever present, exhibited aberrant morphogenesis. F-actin organization was also affected, since the stomatal complex-specific arrays were scarcely observed. At late developmental stages, the overall F-actin network was diminished in all epidermal cells, although thick actin bundles persisted. Taken together, stomatal complex development strongly depends on cell wall mechanical properties. Moreover, F-actin organization exhibits a tight relationship with the cell wall.
Keywords: actin; cell wall; cellulose synthesis; cytoskeleton; leaf epidermis; maize; stomata.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

