The enteric microbiota regulates jejunal Paneth cell number and function without impacting intestinal stem cells
- PMID: 29883265
- PMCID: PMC6363071
- DOI: 10.1080/19490976.2018.1474321
The enteric microbiota regulates jejunal Paneth cell number and function without impacting intestinal stem cells
Abstract
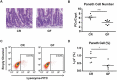
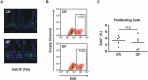
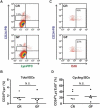
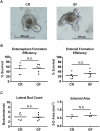
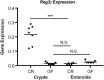
Paneth cells (PCs) are epithelial cells found in the small intestine, next to intestinal stem cells (ISCs) at the base of the crypts. PCs secrete antimicrobial peptides (AMPs) that regulate the commensal gut microbiota. In contrast, little is known regarding how the enteric microbiota reciprocally influences PC function. In this study, we sought to characterize the impact of the enteric microbiota on PC biology in the mouse small intestine. This was done by first enumerating jejunal PCs in germ-free (GF) versus conventionally raised (CR) mice. We next evaluated the possible functional consequences of altered PC biology in these experimental groups by assessing epithelial proliferation, ISC numbers, and the production of AMPs. We found that PC numbers were significantly increased in CR versus GF mice; however, there were no differences in ISC numbers or cycling activity between groups. Of the AMPs assessed, only Reg3γ transcript expression was significantly increased in CR mice. Intriguingly, this increase was abrogated in cultured CR versus GF enteroids, and could not be re-induced with various bacterial ligands. Our findings demonstrate the enteric microbiota regulates PC function by increasing PC numbers and inducing Reg3γ expression, though the latter effect may not involve direct interactions between bacteria and the intestinal epithelium. In contrast, the enteric microbiota does not appear to regulate jejunal ISC census and proliferation. These are critical findings for investigators using GF mice and the enteroid system to study PC and ISC biology.
Keywords: Paneth cell; Reg3γ; enteroid; germ-free; intestinal stem cell; microbiota.
Figures

Similar articles
-
Paneth cells promote angiogenesis and regulate portal hypertension in response to microbial signals.J Hepatol. 2020 Sep;73(3):628-639. doi: 10.1016/j.jhep.2020.03.019. Epub 2020 Mar 20. J Hepatol. 2020. PMID: 32205193
-
Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract.BMC Immunol. 2008 Jul 17;9:37. doi: 10.1186/1471-2172-9-37. BMC Immunol. 2008. PMID: 18637162 Free PMC article.
-
Paneth Cells during Viral Infection and Pathogenesis.Viruses. 2018 Apr 26;10(5):225. doi: 10.3390/v10050225. Viruses. 2018. PMID: 29701691 Free PMC article. Review.
-
Lipid malabsorption from altered hormonal signaling changes early gut microbial responses.Am J Physiol Gastrointest Liver Physiol. 2018 Oct 1;315(4):G580-G591. doi: 10.1152/ajpgi.00135.2018. Epub 2018 Jun 28. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29953253 Free PMC article.
-
Paneth cells: maestros of the small intestinal crypts.Annu Rev Physiol. 2013;75:289-311. doi: 10.1146/annurev-physiol-030212-183744. Annu Rev Physiol. 2013. PMID: 23398152 Review.
Cited by
-
Transplantion of predominant Lactobacilli from native hens to commercial hens could indirectly regulate their ISC activity by improving intestinal microbiota.Microb Biotechnol. 2022 Apr;15(4):1235-1252. doi: 10.1111/1751-7915.13917. Epub 2021 Sep 18. Microb Biotechnol. 2022. PMID: 34536334 Free PMC article.
-
Single-cell view into the role of microbiota shaping host immunity in the larynx.iScience. 2024 May 31;27(6):110156. doi: 10.1016/j.isci.2024.110156. eCollection 2024 Jun 21. iScience. 2024. PMID: 38974468 Free PMC article.
-
Effect of the Microbiome on Intestinal Innate Immune Development in Early Life and the Potential Strategy of Early Intervention.Front Immunol. 2022 Jul 19;13:936300. doi: 10.3389/fimmu.2022.936300. eCollection 2022. Front Immunol. 2022. PMID: 35928828 Free PMC article. Review.
-
The Interplay between Nutrition, Innate Immunity, and the Commensal Microbiota in Adaptive Intestinal Morphogenesis.Nutrients. 2021 Jun 26;13(7):2198. doi: 10.3390/nu13072198. Nutrients. 2021. PMID: 34206809 Free PMC article. Review.
-
A systematic framework for understanding the microbiome in human health and disease: from basic principles to clinical translation.Signal Transduct Target Ther. 2024 Sep 23;9(1):237. doi: 10.1038/s41392-024-01946-6. Signal Transduct Target Ther. 2024. PMID: 39307902 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
