Immune History and Influenza Vaccine Effectiveness
- PMID: 29883414
- PMCID: PMC6027411
- DOI: 10.3390/vaccines6020028
Immune History and Influenza Vaccine Effectiveness
Abstract
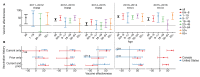
The imperfect effectiveness of seasonal influenza vaccines is often blamed on antigenic mismatch, but even when the match appears good, effectiveness can be surprisingly low. Seasonal influenza vaccines also stand out for their variable effectiveness by age group from year to year and by recent vaccination status. These patterns suggest a role for immune history in influenza vaccine effectiveness, but inference is complicated by uncertainty about the contributions of bias to the estimates themselves. In this review, we describe unexpected patterns in the effectiveness of seasonal influenza vaccination and explain how these patterns might arise as consequences of study design, the dynamics of immune memory, or both. Resolving this uncertainty could lead to improvements in vaccination strategy, including the use of universal vaccines in experienced populations, and the evaluation of vaccine efficacy against influenza and other antigenically variable pathogens.
Keywords: imprinting; original antigenic sin; repeat vaccination; seasonal influenza vaccine; test-negative design; universal influenza vaccine; vaccine effectiveness.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the writing of the manuscript or in the decision to publish.
Figures
References
-
- Belongia E.A., Kieke B.A., Donahue J.G., Greenlee R.T., Balish A., Foust A., Lindstrom S., Shay D.K. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J. Infect. Dis. 2009;199:159–167. doi: 10.1086/595861. - DOI - PubMed
-
- Zost S.J., Parkhouse K., Gumina M.E., Kim K., Perez S.D., Wilson P.C., Treanor J.J., Sant A.J., Cobey S., Hensley S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA. 2017;114:12578–12583. doi: 10.1073/pnas.1712377114. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources