Adjuvant chemotherapy with S-1 after curative chemoradiotherapy in patients with locoregionally advanced squamous cell carcinoma of the head and neck: Reanalysis of the ACTS-HNC study
- PMID: 29883463
- PMCID: PMC5993254
- DOI: 10.1371/journal.pone.0198391
Adjuvant chemotherapy with S-1 after curative chemoradiotherapy in patients with locoregionally advanced squamous cell carcinoma of the head and neck: Reanalysis of the ACTS-HNC study
Abstract
Background: Chemoradiotherapy (CRT) has improved organ preservation or overall survival (OS) of locoregionally advanced head and neck squamous cell cancer (LAHNSCC), but in clinical trials of conventional CRT, increasing CRT intensity has not been shown to improve OS. In the Adjuvant ChemoTherapy with S-1 after curative treatment in patients with Head and Neck Cancer (ACTS-HNC) phase III study, OS of curative locoregional treatments improved more with adjuvant chemotherapy with S-1 (tegafur gimeracil oteracil potassium) than with tegafur/uracil (UFT). ACTS HNC study showed the significant efficacy of S-1 after curative radiotherapy in sub-analysis. We explored the efficacy of S-1 after curative CRT in a subset of patients from the ACTS-HNC study.
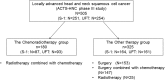
Methods: Patients with stage III, IVA, or IVB LAHNSCC were enrolled in this study to evaluate the efficacy of S-1 compared with UFT as adjuvant chemotherapy after curative CRT in the ACTS-HNC study. Patients received S-1 at 80-120 mg/day in two divided doses for 2 weeks, followed by a 1-week rest, or UFT 300 or 400 mg/day in two or three divided doses daily, for 1 year. The endpoints were OS, disease-free survival, locoregional relapse-free survival, distant metastasis-free survival (DMFS), and post-locoregional relapse survival.
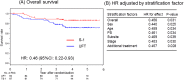
Results: One hundred eighty patients (S-1, n = 87; UFT, n = 93) were included in this study. Clinical characteristics of the S-1 and UFT arms were similar. S-1 after CRT significantly improved OS (hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.22-0.93) and DMFS (HR, 0.50; 95% CI, 0.26-0.97) compared with UFT.
Conclusion: As adjuvant chemotherapy, S-1 demonstrated better efficacy for OS and DMFS than UFT in patients with LAHNSCC after curative CRT and may be considered a treatment option following curative CRT. For this study was not preplanned in the ACTS-HNC study, the results is hypothesis generating but not definitive.
Conflict of interest statement
A. Kubota has received honoraria from AstraZeneca, Otsuka Pharmaceutical, and Taiho Pharmaceutical; and research funding from Taiho. K. Tsukahara has received honoraria from Covidien Japan, FUJIFILM Medical, Johnson & Johnson, KYORIN Pharmaceutical, Kyowa Hakko Kirin, Merck Serono, Mitsubishi Tanabe Pharma Corporation, and Sanofi. T. Terada has received honoraria from GlaxoSmithKline, KYORIN, Merck Serono, and Ono Pharmaceutical. T. Taguchi has received an honorarium from Taiho. K. Nagahara has received honoraria from Kowa Pharmaceutical and Taisho Toyama Pharmaceutical. S. Iwae has received honoraria from Ono and Otsuka. K. Tomita has received an honorarium from KYORIN. S. Teramukai has received consulting fees from Bristol-Myers Squibb, Daiichi Sankyo, Sanofi, Sysmex Corporation, and Taiho; and research funding from Daiichi Sankyo. M. Fujii has received honoraria from Bristol-Myers Squibb, Merck Serono, and Taiho; and research funding from Taiho. The other authors have declared no conflicts of interest. We confirm that our potential interests do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Randomized phase III trial of adjuvant chemotherapy with S-1 after curative treatment in patients with squamous-cell carcinoma of the head and neck (ACTS-HNC).PLoS One. 2015 Feb 11;10(2):e0116965. doi: 10.1371/journal.pone.0116965. eCollection 2015. PLoS One. 2015. PMID: 25671770 Free PMC article. Clinical Trial.
-
A randomized phase III trial comparing S-1 versus UFT as adjuvant chemotherapy for stage II/III rectal cancer (JFMC35-C1: ACTS-RC).Ann Oncol. 2016 Jul;27(7):1266-72. doi: 10.1093/annonc/mdw162. Epub 2016 Apr 7. Ann Oncol. 2016. PMID: 27056996 Free PMC article. Clinical Trial.
-
[A randomized controlled trial to evaluate the effect of adjuvant oral fluoropyrimidine derivative therapy after curative resection for stage II/III rectal cancer-adjuvant chemotherapy trial of S-1 for rectal cancer (ACTS-RC):].Gan To Kagaku Ryoho. 2006 Jun;33 Suppl 1:138-43. Gan To Kagaku Ryoho. 2006. PMID: 16897990 Clinical Trial. Japanese.
-
[Adjuvant chemotherapy with S-1 for advanced head and neck carcinoma].Gan To Kagaku Ryoho. 2006 Jun;33 Suppl 1:172-8. Gan To Kagaku Ryoho. 2006. PMID: 16897997 Review. Japanese.
-
Future directions in the treatment of squamous cell carcinoma of the head and neck: the role of UFT.Oncology (Williston Park). 1997 Sep;11(9 Suppl 10):86-9. Oncology (Williston Park). 1997. PMID: 9348576 Review.
Cited by
-
Current status and future perspective of postoperative treatment for locally advanced squamous cell carcinoma of the head and neck.Jpn J Clin Oncol. 2024 Jun 1;54(6):613-619. doi: 10.1093/jjco/hyae029. Jpn J Clin Oncol. 2024. PMID: 38452121 Free PMC article. Review.
-
Phase II trial of combination treatment with S-1/cetuximab in patients with platinum-ineligible recurrent and/or metastatic squamous cell carcinoma of the head and neck.Int J Clin Oncol. 2021 Jan;26(1):51-58. doi: 10.1007/s10147-020-01788-6. Epub 2020 Sep 29. Int J Clin Oncol. 2021. PMID: 32996023 Clinical Trial.
-
Prognostic Biomarkers of Salvage Chemotherapy Following Nivolumab Treatment for Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma.Cancers (Basel). 2020 Aug 15;12(8):2299. doi: 10.3390/cancers12082299. Cancers (Basel). 2020. PMID: 32824226 Free PMC article.
References
-
- Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, Laramore GE, et al.; Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–90. doi: 10.1056/NEJM199106133242402 - DOI - PubMed
-
- Lefèbvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890–9. - PubMed
-
- Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiation versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–7. doi: 10.1200/JCO.1998.16.4.1310 - DOI - PubMed
-
- Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–6. - PubMed
-
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

